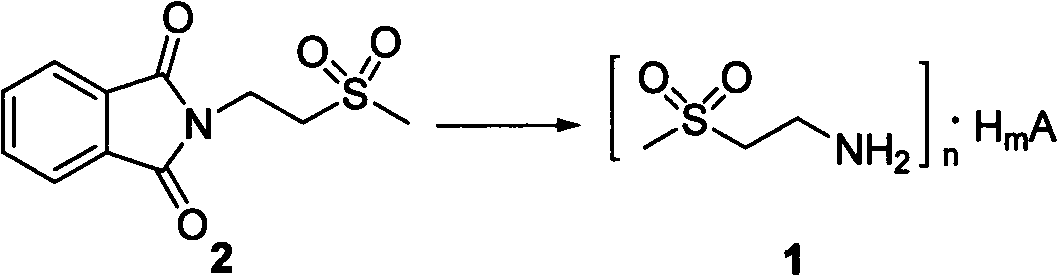
Preparation methods of 2-(amino)ethyl methyl sulfone salt and intermediate of 2-(amino)ethyl methyl sulfone salts
A technology of ethyl methyl sulfone salt and amino group, which is applied in the field of preparation of 2-ethyl methyl sulfone salt and its intermediates, which can solve the problems of large environmental pollution and unfavorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthesis of embodiment 1 lapatinib
[0035] Add 5-[4-[[3-chloro-4-[(3-fluorophenyl)methoxy]phenyl]amino]-6-quinazoline]-2-furancarbaldehyde (4.73 g, 10mmol), 2-(amino)ethylmethylsulfone hydrochloride (2.56g, 16mmol), diisopropylethylamine (5.17g, 40mmol), acetic acid (2.4g, 40mmol) and 50ml tetrahydrofuran, Stir the reaction at room temperature for 3 h, add sodium triacetoxyborohydride (8.48 g, 40 mmol) in batches, and react at room temperature for 12 h. Add 30ml of 20% aqueous sodium hydroxide solution and stir to separate the layers, and extract the inorganic phase with ethyl acetate. Combine the organic phases, concentrate, add 50ml of THF to the residue to dissolve, add p-toluenesulfonic acid aqueous solution (7.6g, 40mmol / 15ml), stir at room temperature for 12h, filter, and dry the filter cake to obtain 7.58g of light yellow solid, melting point 244-246°C , yield 82.8%, HPLC purity: 99.6%.
[0036] The structural identification data are as follows: HNMR (DM...
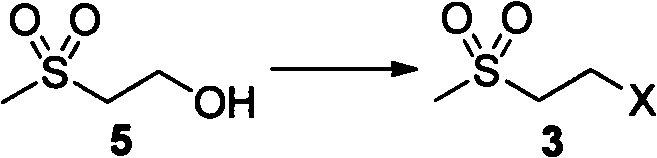
Embodiment 2
[0037] The synthesis of embodiment 22-chloroethyl methyl sulfone
[0038] Add 2-hydroxyethyl sulfone (6.21g, 50mmol), pyridine (3.9g, 50mmol) and 50ml toluene into a 250ml reaction flask, slowly add thionyl chloride (17.8g, 0.15mol) dropwise, and heat to reflux after dropping 2h, cooling. Add 50 ml of ice water and stir, separate the liquids, extract the aqueous phase with dichloromethane, combine the organic phases, dry and concentrate to obtain 5.9 g of brown oil, which can be directly used in the next step reaction with a yield of 83.1%.
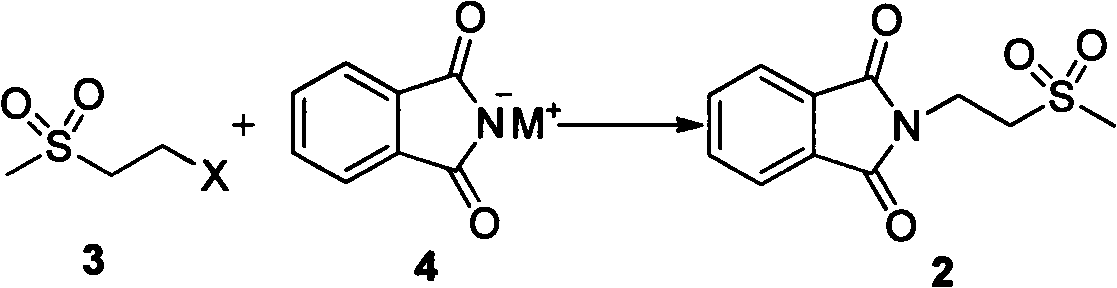
Embodiment 3
[0039] Synthesis of Example 32-(2-thiamphenicol ethyl)isoindole-1,3-dione
[0040] Add potassium phthalimide (5.56g, 30mmol), sodium iodide (0.42g, 2.8mmol) and 30ml of DMF in a 250ml reaction flask, raise the temperature to 100°C, and add 2-chloroethylmethyl sulfone ( 4.2g, 30mmol), keep warm at 100°C for 2.5h, and cool. Add 50ml of water and stir for 1 hour, filter, and dry the filter cake to obtain 6.75g of white solid with a melting point of 172-174°C and a yield of 88.8%.
[0041] The structural identification data are as follows: H-NMR (CDCl 3 ): δ7.88(m, 2H), 7.75(m, 2H), 4.20(t, 2H), 3.43(2, 1H), 3.03(s, 3H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com