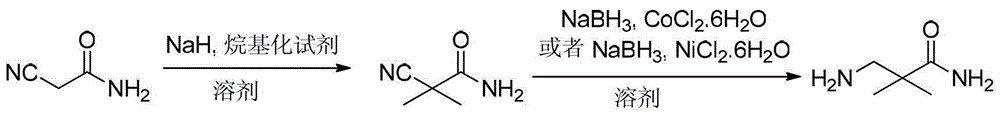
Preparation method for 3-amino-2, 2-dimethylpropionamide
A technology of dimethylpropionamide and dimethylcyanoacetamide, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of increasing the cost and danger of production, and achieve the effects of simple operation, environmental protection and low preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A) Preparation of dimethylcyanoacetamide:
[0026] Add cyanoacetamide into the reaction vessel (with a stirring device) filled with organic solvent, that is, tetrahydrofuran, wherein: the weight ratio of tetrahydrofuran to cyanoacetamide is 30:1, and stir for 30 minutes at a temperature of -5°C , after stirring and dissolving, add sodium hydride with a mass percentage concentration of 60%, and stir the reaction for 120min under the condition of controlling the temperature at -5°C. After the stirring reaction is completed, slowly add dimethyl sulfate for alkylation reaction. The temperature of the alkylation reaction is 20°C, and the time of the alkylation reaction is 12h. After the alkylation reaction is completed, add saturated NH 4 Cl aqueous solution, distill off the tetrahydrofuran solvent, then extract with ethyl acetate, wash with water, and spin-dry (spin dry) to obtain the primary product of dimethyl cyanoacetamide in the form of light yellow solid powder, that ...
Embodiment 2
[0030] A) Preparation of dimethylcyanoacetamide:
[0031] Add cyanoacetamide in the reaction vessel (with stirring device) filled with organic solvent, that is, 2-methyltetrahydrofuran, wherein: the weight ratio of 2-methyltetrahydrofuran to cyanoacetamide is 50:1, in Stir at -5°C for 30 minutes, add sodium hydride with a mass percentage concentration of 60% after stirring and dissolving, and stir and react for 30 minutes at a controlled temperature of 10°C. After the stirring reaction is completed, slowly add diethyl sulfate dropwise Carry out the alkylation reaction, the temperature of the alkylation reaction is 0°C, and the time of the alkylation reaction is 24h. After the alkylation reaction is completed, add saturated NH 4 Cl aqueous solution, distill off the tetrahydrofuran solvent, then extract with ethyl acetate, wash with water, and spin-dry (spin dry) to obtain the primary product of dimethyl cyanoacetamide in the form of light yellow solid powder, that is, to obtain...
Embodiment 3
[0036] A) Preparation of dimethylcyanoacetamide:
[0037] Add cyanoacetamide in the reaction vessel (with stirring device) filled with organic solvent, that is, 2-methyltetrahydrofuran, wherein: the weight ratio of 2-methyltetrahydrofuran to cyanoacetamide is 10:1, in Stir at -5°C for 30 minutes, add sodium hydride with a mass percentage concentration of 60% after stirring and dissolving, and stir for 180 minutes at a controlled temperature of -10°C. After the stirring reaction is completed, slowly add methyl iodide dropwise to carry out Alkylation reaction, the temperature of the alkylation reaction is 10°C, the time of the alkylation reaction is 8h, and saturated NH 4 Cl aqueous solution, distill off the tetrahydrofuran solvent, then extract with ethyl acetate, wash with water, and spin-dry (spin dry) to obtain the primary product of dimethyl cyanoacetamide in the form of light yellow solid powder, that is, to obtain crude dimethyl cyanoacetamide Acetamide, then recrystalli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com