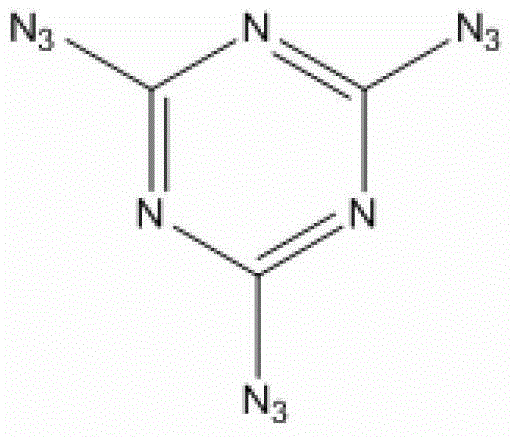
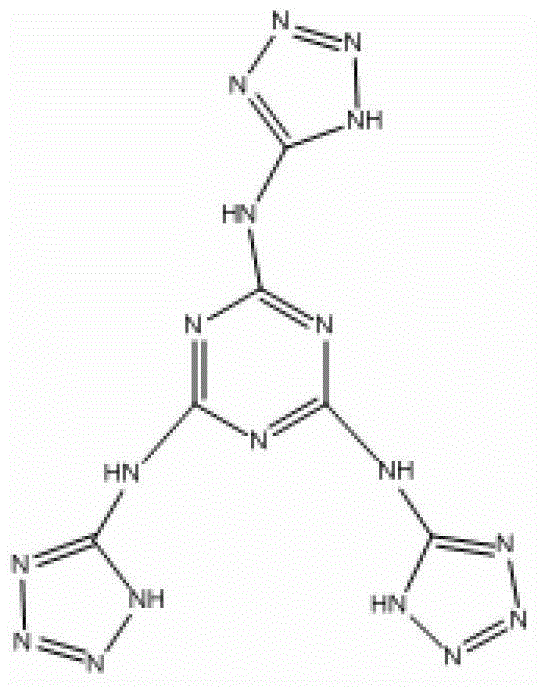
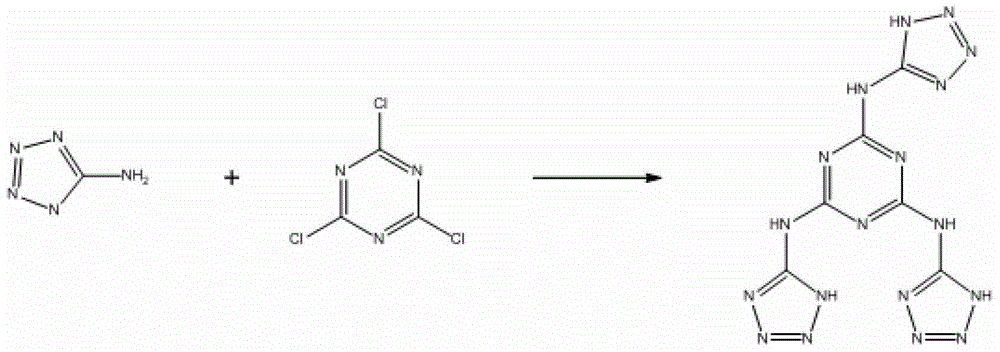
2,4,6-tri(5-amino tetrazole)-1,3,5-triazine compound and preparation method thereof
A technology of triazine compound and aminotetrazole, which is applied in the field of high nitrogen-containing energy compounds, can solve the problems of poor thermal stability, limited application of triazine compounds, poor stability, etc., and achieves the effect of good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 1.404g (16.7mmol) of 5-aminotetrazole and 1.2mL of triethylamine into 100mL of acetonitrile to dissolve. In the reaction bottle, a white solid gradually dissolves in acetonitrile. After adding triethylamine, there are a small amount of air bubbles in the reaction bottle emerge;
[0022] At 0°C, add 0.512g (2.78mmol) 2,4,6-trichloro-1,3,5-triazine, use an ice bath to control the reaction temperature at 0-10°C during the addition, and complete the addition Withdraw the ice bath and react for 2 hours, and then raise the temperature to reflux temperature for reaction. During this process, most of the added 2,4,6-trichloro-1,3,5-triazine began to be suspended on the reaction solution. As the process progresses, the suspended solids of 2,4,6-trichloro-1,3,5-triazine gradually decrease, and a finer white powder is formed thereupon. The progress of the reaction was monitored by thin-layer chromatography. After 6 hours, the point of the raw material 2,4,6-trichloro-1,3,5-tr...
Embodiment 2
[0036] Add 1.386g (16.5mmol) 5-aminotetrazole and 2.0mL ammonia water into 100mL acetonitrile to dissolve;
[0037] At 0°C, add 0.506g (2.74mmol) 2,4,6-trichloro-1,3,5-triazine, and use an ice bath to control the reaction temperature at 0-10°C during the addition process. , reacted for 2 hours after removing the ice bath, then heated up to reflux reaction, and used thin layer chromatography to monitor the progress of the reaction. After 6 hours, the point of the raw material 2,4,6-trichloro-1,3,5-triazine disappeared, and the Remove the heating apparatus to end the reaction;
[0038] After the reaction was completed, the acetonitrile solution was removed in vacuo after the reaction liquid was lowered to 15°C, and the obtained solids were successively washed with 100 mL of dilute hydrochloric acid with a mass concentration of 5%, 120 mL of ethanol and 100 mL of acetone, filtered, and dried to obtain a white solid product 0.402 g, 44.0% yield, 97.0% purity (HPLC).
[0039] The...
Embodiment 3
[0042] Add 1.458g (17.4mmol) of 5-aminotetrazole and 1.2mL of triethylamine into 100mL of tetrahydrofuran to dissolve;
[0043] At 0°C, add 0.523g (2.84mmol) 2,4,6-trichloro-1,3,5-triazine, and use an ice bath to control the reaction temperature at 0-10°C during the addition process. , reacted for 2 hours after removing the ice bath, then heated up to reflux reaction, and used thin layer chromatography to monitor the progress of the reaction. After 6 hours, the point of the raw material 2,4,6-trichloro-1,3,5-triazine disappeared, and the Remove the heating apparatus to end the reaction;
[0044] After the reaction was completed, when the reaction liquid was lowered to 15°C, the acetonitrile solution was removed in vacuo, and the obtained solids were successively washed with 100 mL of dilute hydrochloric acid with a mass concentration of 5%, 120 mL of ethanol, and 100 mL of acetone, filtered, and dried to obtain a white solid product of 0.389 g, 41.2% yield, 97.1% purity (HPLC...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| NMR spectroscopy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com