Triblock polycation, and preparation method and application thereof
A polycationic, tri-block technology, applied in the field of medicine and chemical industry, can solve the problems of rapid dissociation and release of entrapped drugs, limited application range and effect, lack of selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the preparation of polyethylene glycol initiator
[0045] Weigh 15.0 g of methoxy-terminated polyethylene glycol 2000 (mPEG 2k -OH) was dissolved in 187.5 ml of dichloromethane, and 0.825 ml of triethylamine was added to the solution. Take 1.5 ml of 2-bromoisobutyryl bromide and dissolve it in 45 ml of dichloromethane, and add the 2-bromoisobutyryl bromide solution dropwise to mPEG under ice-cooling 2k -OH solution. After the dropwise addition, the reaction was continued for 48 hours, washed twice with saturated sodium bicarbonate and saturated saline, the organic phase was collected, dried overnight with anhydrous magnesium sulfate, filtered to remove magnesium sulfate, concentrated by rotary evaporation, and precipitated with diethyl ether twice to obtain acyl Bromine-modified polyethylene glycol polymer product 11.0 grams, yield 73.3%.
Embodiment 2
[0046] Embodiment 2: Preparation of polyethylene glycol-polyglycidyl methacrylate diblock copolymer
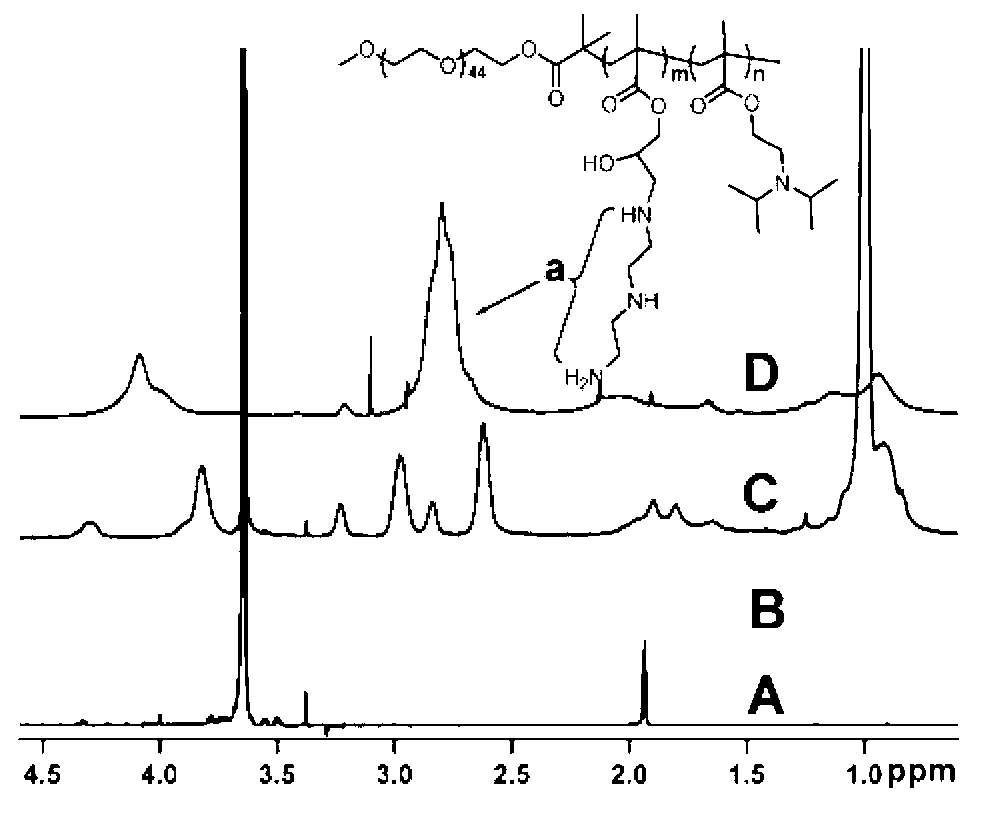
[0047] Get the macromolecular initiator (mPEG) of polyethylene glycol 2000 prepared in embodiment 1 2k -Br) 1.0 g was dissolved in 3.6 ml of N,N-dimethylacetamide (DMAC), 0.4 ml of isopropanol was added, stirred and mixed, and 1.05 ml of glycidyl methacrylate (GC) was added, pentamethyl Diethylenetriamine 42 µl. After deoxygenation in vacuum, 4.8 mg of cuprous chloride catalyst was added and reacted at 40°C for 15 hours. After the reaction was completed, it was purified with an alumina column, concentrated by rotary evaporation, precipitated with ether, and dried with a vacuum pump to obtain 1.44 g of the product, with a yield of 72%. Using deuterated chloroform as a solvent, the polymer structure was determined by H NMR spectroscopy as methoxy-terminated polyethylene glycol-polyglycidyl methacrylate (mPEG 2k -b-PGC 40 ).
Embodiment 3
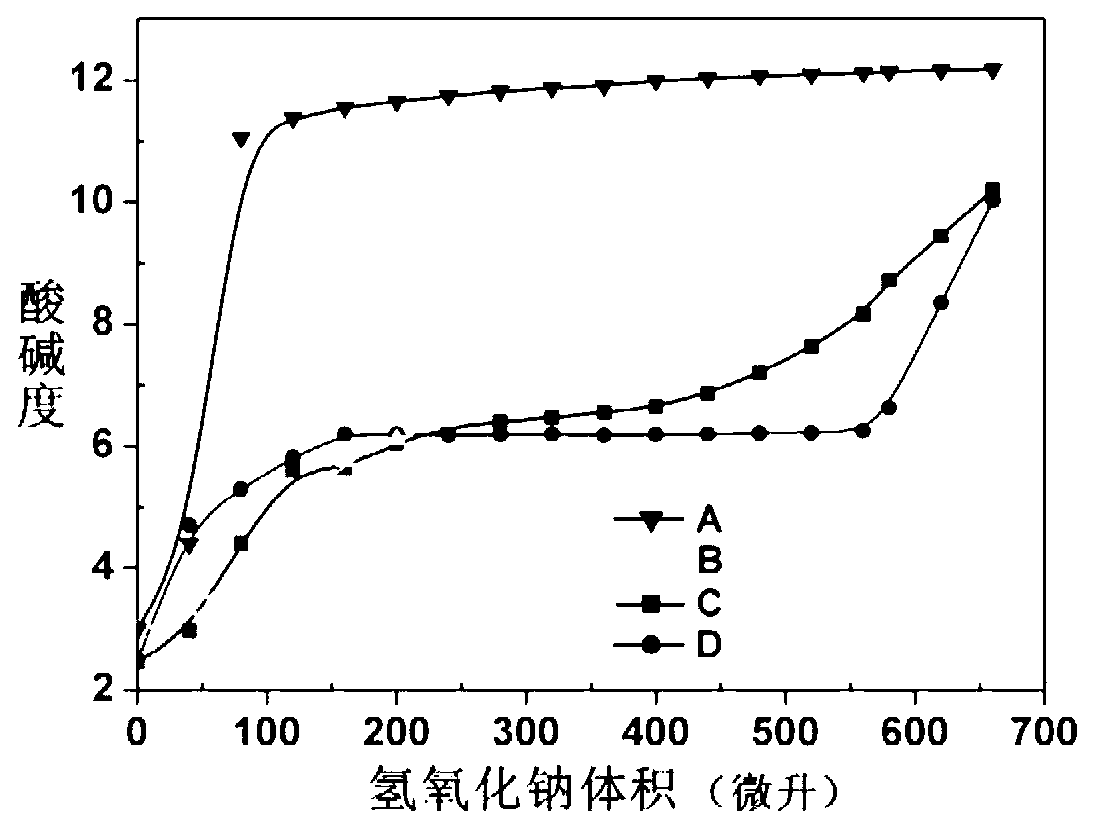
[0048] Embodiment 3: Preparation of polyethylene glycol-polyglycidyl methacrylate-polydiisopropylaminoethyl methacrylate triblock copolymer
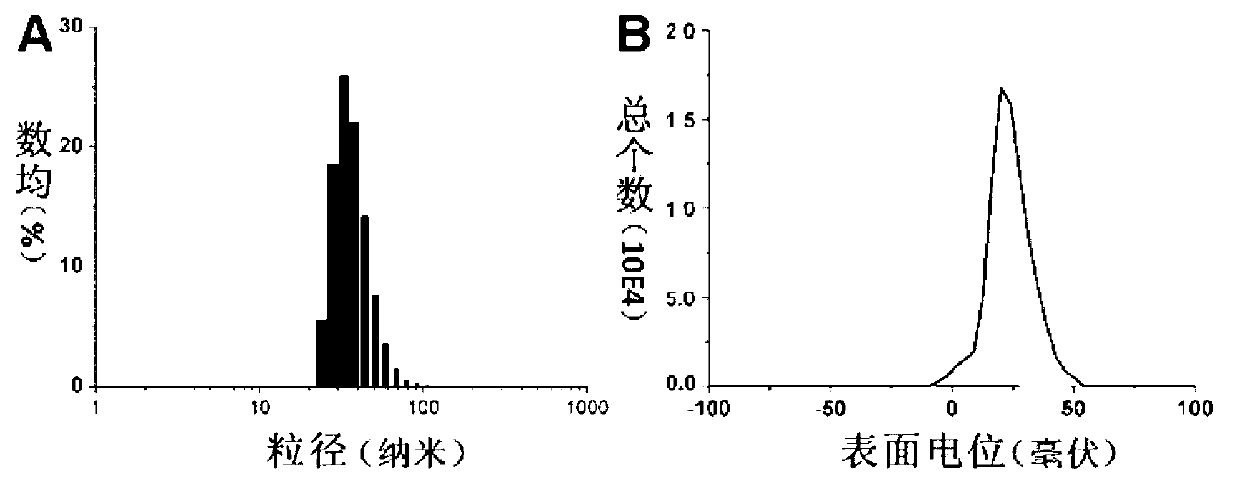
[0049] Get the methoxy-terminated polyethylene glycol-polyglycidylmethacrylate (mPEG) prepared in Example 2 2k -b-PGC 40 ) 0.2 g was dissolved in 0.3 ml of DMAC, 0.2 ml of isopropanol was added, stirred and mixed, and then 0.47 ml of diisopropylaminoethyl methacrylate monomer and 7.5 μl of pentamethyldiethylenetriamine were added. After deoxygenation, 5.0 mg of cuprous bromide catalyst was added, and reacted at 40°C for 48 hours. After the reaction was completed, the copper salt was removed through an aluminum oxide column, concentrated by rotary evaporation at 40°C, precipitated with ether, and dried in vacuum to obtain 2.0 g of the product, with a yield of 98%. Using deuterated chloroform as a solvent, the polymer structure was confirmed by hydrogen nuclear magnetic resonance as polyethylene glycol-polyglycidyl methacrylate-polydiiso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com