Preparation method of triblock acrylic acid ester thermoplastic elastomer
A thermoplastic elastomer and acrylate technology, which is applied in the field of three-block thermoplastic elastomers, can solve the problems of difficult removal and reuse of catalysts, difficulty in industrialization, and increased production costs, so as to improve heat aging resistance and chemical Responsiveness, low catalyst usage, and reduced sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
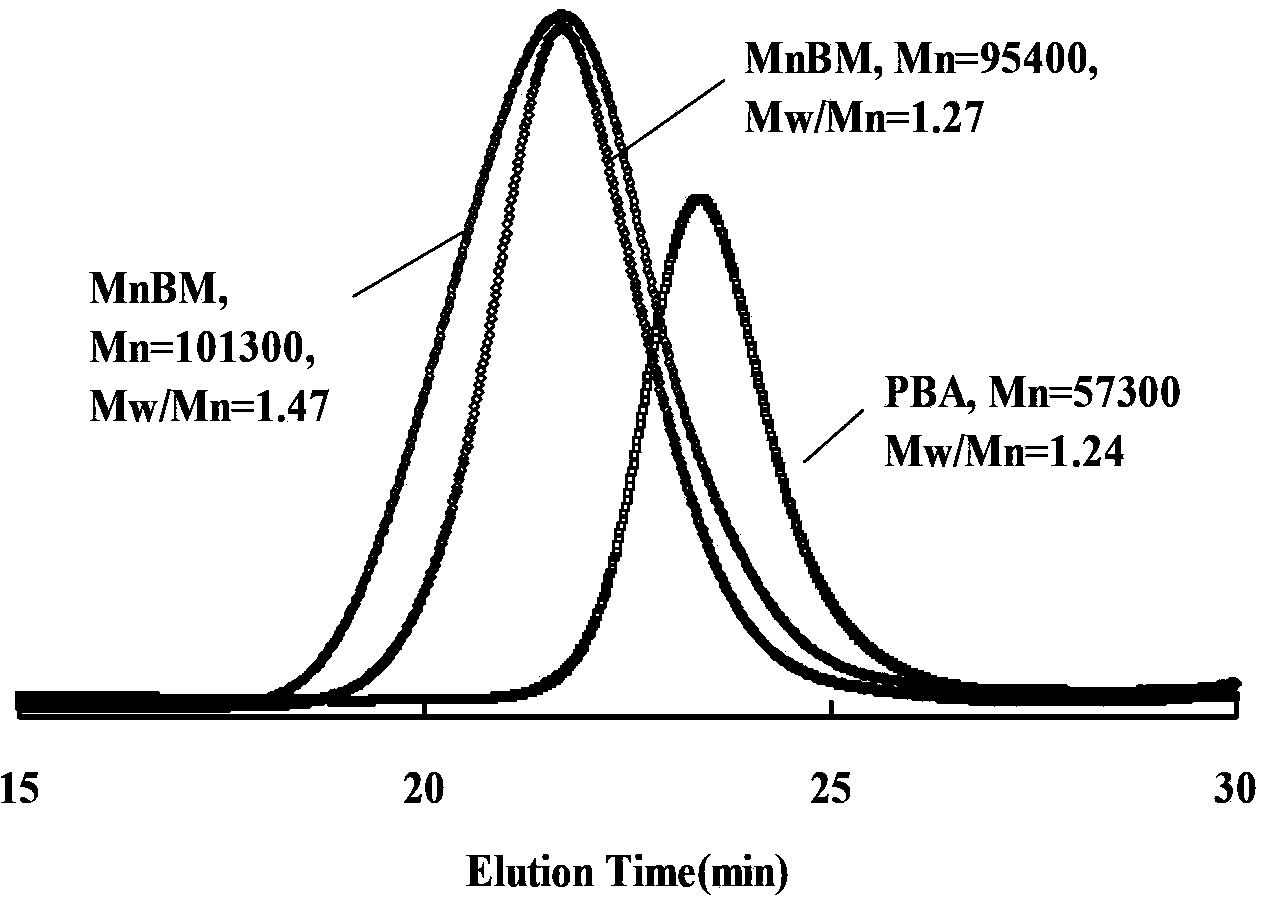
[0027] (1) Preparation of bifunctional macroinitiator polybutylacrylate: preset molecular weight 60000 (initiator:monomer=1:600,
[0028] Conversion rate 80% stop reaction)
[0029] Add 76.90g (0.6mol) monomer BA, 0.3880g (1×10 -3 mol) difunctional initiator diα-bromoisobutyrate 1,4-butanediol, 0.02680g (1.2×10 -4 mol) Catalyst CuBr 2 , 0.3122g (1.8×10 - 3mol) ligand PMDETA, 0.9720g (2.4×10 -3mol) reducing agent Sn(EH)2, 15.23g toluene, 5g anisole, mix evenly, then vacuumize and fill with nitrogen, heat up and stir, and react at 75°C. Samples were taken every 30 minutes, and the monomer conversion rate was measured by gas chromatography and the molecular weight and molecular weight distribution were measured by gel permeation chromatography. After 12 hours of reaction, the conversion rate reached 79.5%, and the reaction ended. The solvent in the system was distilled off under reduced pressure to obtain a light yellow transparent viscous product. The product quality is 5...
Embodiment 2
[0036] (1) Use the same difunctional macromolecular initiator as in Example 1.
[0037] (2) Preparation of triblock copolymer poly(methyl methacrylate-b-butyl acrylate-b-methyl methacrylate): preset
[0038] Molecular weight 100000 (initiator:monomer=1:500, conversion rate 90% stop reaction)
[0039] In a 100mL four-neck flask with a thermometer, add 10.07g (0.1mol) monomeric methyl methacrylate (MMA), 10.65g (2×10 - 4mol) polybutylacrylate (PBA) macroinitiator, 0.002690g (2×10 -5 mol) Catalyst CuCl 2 , 0.3469g (3×10 -5 mol) Ligand PMDETA, 0.0081g (2×10 -5 mol) reducing agent Sn(EH)-2, 15.57g toluene, 5.13g anisole, mixed evenly, then evacuated and filled with nitrogen, heated and stirred, and reacted at 75°C. Samples were taken every 30 minutes, and the monomer conversion rate was measured by gas chromatography and the molecular weight and molecular weight distribution were measured by gel permeation chromatography. After 8.5 hours of reaction, the conversion rate reach...
Embodiment 3
[0043] (1) Preparation of bifunctional macromolecular initiator polybutylacrylate: preset molecular weight 2000 (initiator:monomer=1:20, conversion rate 80% stop reaction)
[0044] Add 76.78g (0.6mol) monomer BA, 10.8g (0.03mol) bifunctional initiator α-bromoisobutyrate 1,4-ethylene glycol, 0.04048g in a 250mL four-neck flask with a thermometer (3×10 -4 mol) catalyst CuCl2, 0.7005g (1.5×10 -3 mol) ligand tris-(N,N-dimethylaminoethyl)amine (Me 6 TREN), 0.2645g (1.5×10 -3 mol) ascorbic acid (AA), 15.15gs tetrahydrofuran, 3.20g toluene, mix evenly, then vacuumize and fill with argon, heat up and stir, and react at 60°C. Samples were taken every 30 minutes, and the monomer conversion rate was measured by gas chromatography and the molecular weight and molecular weight distribution were measured by gel permeation chromatography. After 6.5 hours of reaction, the conversion rate reached 85.2%, and the reaction ended. The solvent in the system was distilled off under reduced pres...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com