Methylbenzofuran quinoline derivative, preparation method thereof, and application of derivative as antitumor drug
A technology of furaquinoline and derivatives, which can be used in antitumor drugs, drug combinations, pharmaceutical formulations, etc., can solve problems such as limited resources, and achieve the effects of increasing steric hindrance, increasing groove binding ability, and good inhibitory activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
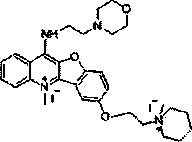
[0043] Embodiment one: the synthesis of compound 8M7
[0044] Dissolve 0.3mol of chloroacetic acid in 60ml of water, adjust the pH to 9 with sodium hydroxide, then add 0.2mol of p-hydroxyanisole, and reflux at 100°C to obtain M1, and then add thionyl chloride for chlorination reaction to obtain M2 , evaporated the thionyl chloride solvent to obtain a brown liquid, and then carried out condensation reaction with anthranilic acid to obtain M3, then preheated PPA to 130°C and added M3 for a compound reaction to obtain compound M4, and mixed M4 with thionyl chloride The chlorination reaction was carried out under reflux at 80°C to obtain compound M5, and then the 7-position methyl group was removed by using boron tribromide in dichloromethane to obtain compound M6.
[0045] Then, under the condition of chloroform (300mL) as solvent, add 6.0g triphenylphosphine, 2.0g M6, 6mL 4-hydroxyethylmorpholine, 6mL diisopropyl azodicarboxylate, N 2 Under protection, diisopropyl azodicarboxyl...
Embodiment 2
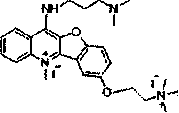
[0049] Embodiment two: the synthesis of compound 9M7
[0050] The method is the same as in Example 1, except that N-hydroxyethylpiperidine is used instead of 4-hydroxyethylmorpholine to obtain compound 9M7.
[0051] Yield: 84%; Melting point: 178.4-180.1°C; 1H NMR (400 MHz, CDCl3) δ 8.36 (dd, J = 8.4, 0.8 Hz, 1H), 8.30 (d, J = 8.5 Hz, 1H), 7.81 ( d, J = 2.6 Hz, 1H), 7.78 (dd, J = 8.4, 1.4 Hz, 1H), 7.70 (dd, J = 11.1, 4.1 Hz, 1H), 7.58 (d, J = 9.0 Hz, 1H), 7.28 (dd, J = 8.8, 2.5 Hz, 1H), 4.27 (t, J = 5.9 Hz, 2H), 2.87 (t, J = 5.9 Hz, 2H), 2.59 (m, 4H), 1.71 – 1.59 (m , 4H), 1.51 – 1.44 (m, 2H); C 22 h 21 ClN 2 o 2 ,LC-MS m / z: 381[M+H] + .
[0052]
[0053] Compound 9M7
Embodiment 3
[0054] Embodiment three: the synthesis of compound 6M8
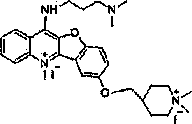
[0055] The method is the same as in Example 1, except that N,N-dimethylethanolamine is used instead of 4-hydroxyethylmorpholine. After the reaction product is separated by a column, the product is added to a 100mL single-necked bottle, and then 15mL of sulfolane is added. React with 10 mL of iodomethane at 68°C for three days, add a large amount of ether, filter, and dry to obtain compound 6M8 as an orange-red solid.
[0056] Yield: 92%; Melting point: 297.2-298.4°C; 1H NMR (400 MHz, DMSO) δ 8.83 (d, J = 9.0 Hz, 1H), 8.59 (d, J = 8.4 Hz, 1H), 8.32 (t, J = 8.0 Hz, 1H), 8.27 (d, J = 2.1 Hz, 1H), 8.20 (d, J = 9.2 Hz, 1H), 8.12 (t, J = 7.8 Hz, 1H), 7.81 (dd, J = 9.2, 2.3 Hz, 1H), 4.93 (s, 3H), 4.75 (t, J=3.6 Hz, 2H), 3.92 (t, J=3.6 Hz, 2H), 3.27 (s, 9H); 21 h 23 ClI 2 N 2 o 2 , ESI-MS m / z: 185 [M-2I] 2+ / 2.
[0057]
[0058] Compound 6M8
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com