Rhodamine fluorescent molecular probe using quinoline derivative as identification group and synthesis method thereof
A technology of fluorescent molecular probe and synthesis method, which is applied in the field of rhodamine-based fluorescent molecular probe and its synthesis, can solve the problem of few probe molecules, and achieve the effects of high selectivity and sensitivity, easy availability of raw materials and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0026] Specific embodiment 1: In this embodiment, a rhodamine-based fluorescent molecular probe using quinoline derivatives as recognition groups has a structural formula:
[0027]
[0028] where the R 1 Is hydrogen, methyl, halogen or 2'-pyridyl; R 2 is hydrogen or halogen.
[0029] This embodiment includes the following beneficial effects:
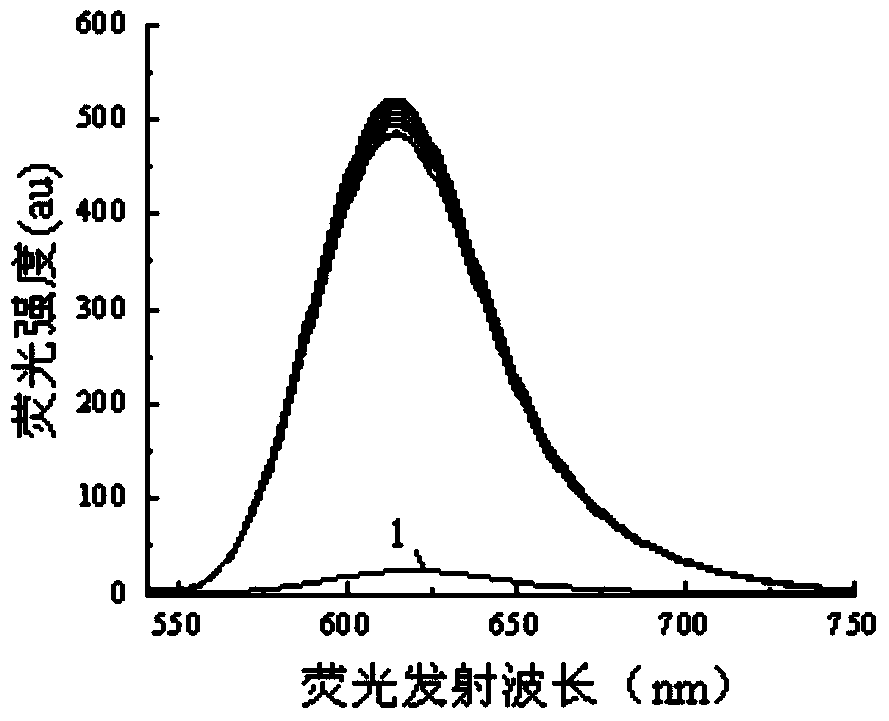
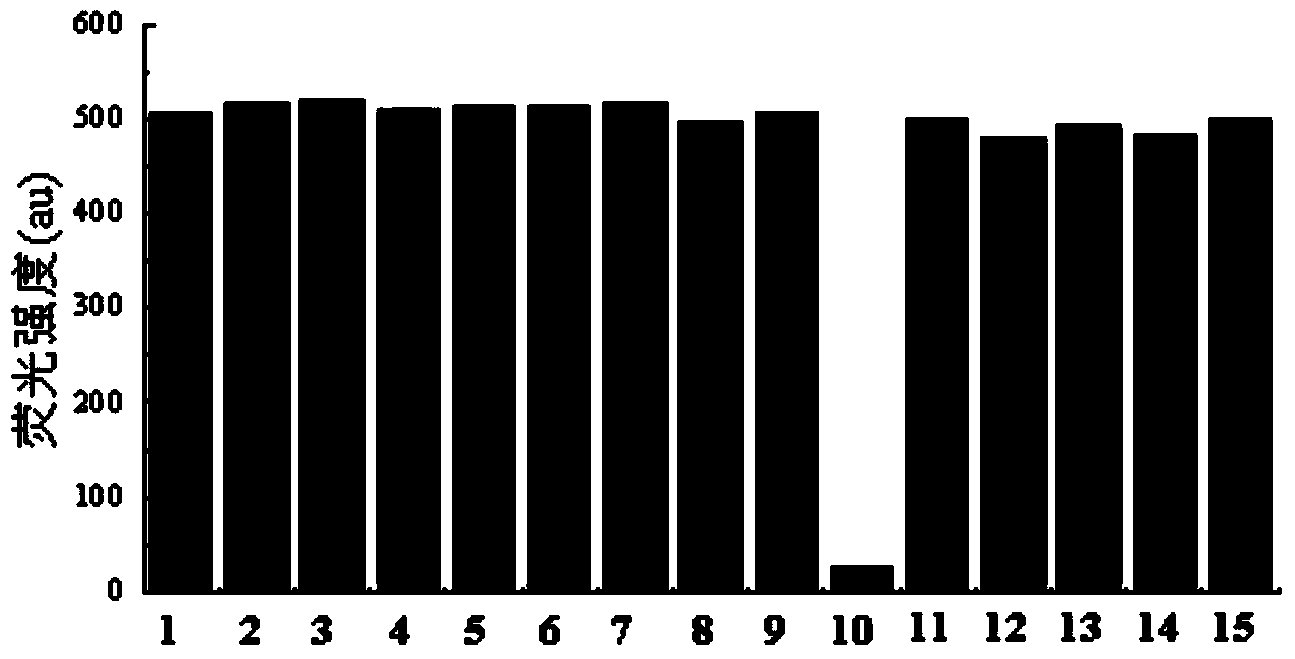
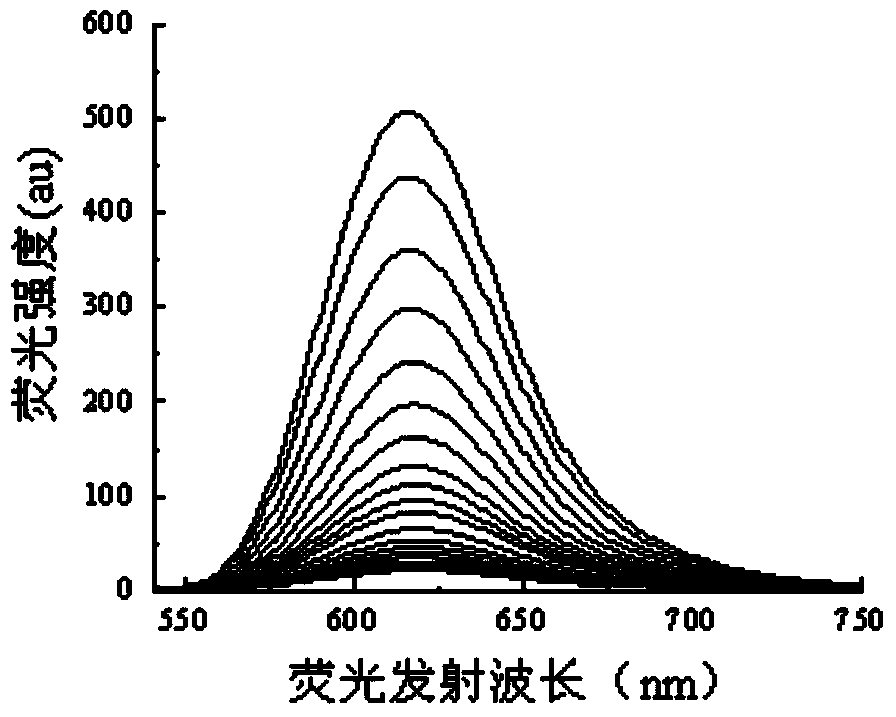
[0030] 1. The fluorescent molecular probe of this embodiment can be directly used for the fluorescence ratio detection of metal ions in water, methanol, DMSO, DMF solvent or their mixed solvents, and has high selectivity and sensitivity;
[0031] 2. The fluorescent molecular probe of this embodiment is easy to synthesize, and the raw materials are easy to get. Among various common metal ions, Cu 2+ Exhibits good selective fluorescence recognition performance;
[0032] 3. The fluorescent molecular probe synthesized in this embodiment can also be used for fluorescence imaging detection of metal ions in biological tissues and cell mi...
specific Embodiment approach 2
[0033] Specific embodiment two: a kind of synthetic method of the rhodamine class fluorescent molecular probe with quinoline derivative as recognition base described in specific embodiment one is to carry out according to the following steps:
[0034] 1. Dissolve 1-5mmol of rhodamine B and 1-5mmol of 8-aminoquinoline derivatives in 10-30mL of chloroform, then add 2-5mL of phosphorus oxychloride, and reflux for 2 ~4h, the reaction solution was obtained;
[0035] 2. Use ammonia water to adjust the pH of the reaction solution obtained in step 1 to 8-10, then spin dry, and then separate through silica gel column chromatography to obtain a rhodamine-based fluorescent molecular probe with quinoline derivatives as recognition groups.
specific Embodiment approach 3
[0036] Specific embodiment three: In this embodiment, a rhodamine-based fluorescent molecular probe with a quinoline derivative as a recognition base has a structural formula:
[0037]
[0038] where the R 3 is hydrogen, acetylamino, α-C 1 ~C 4 Linear alkylaminoacetamido or C 1 ~C 4 Straight-chain alkylsulfonylamino.
[0039] Specific embodiment four: a kind of synthetic method of the rhodamine class fluorescent molecular probe with quinoline derivative as recognition base described in specific embodiment three is to carry out according to the following steps:
[0040] 1. Dissolve 0.2-0.5 mmol of rhodamine B in 2-8 mL of hydrazine hydrate, reflux for 20-40 minutes under the protection of nitrogen, spin the reaction solution to dryness, and then separate through silica gel column chromatography to obtain the intermediate rhodamine B acyl Hydrazine;
[0041] 2. Dissolve 0.1 to 0.8 mmol of the intermediate rhodamine B hydrazide obtained in step 1, and 0.2 to 1.0 mmol of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com