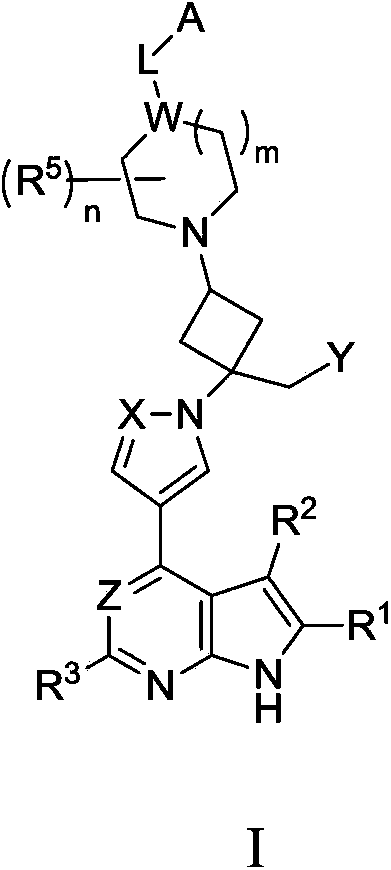
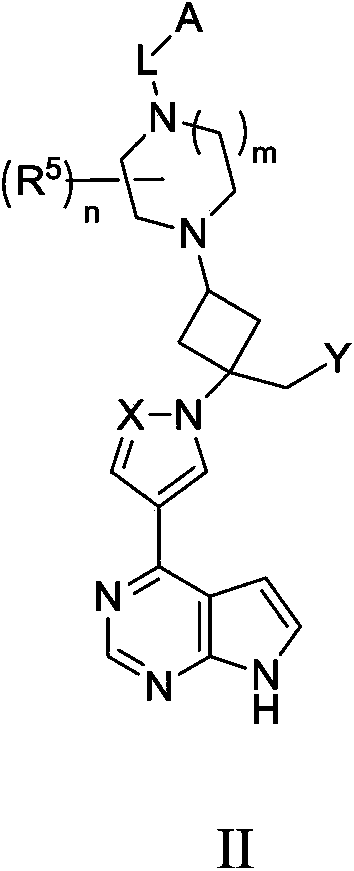
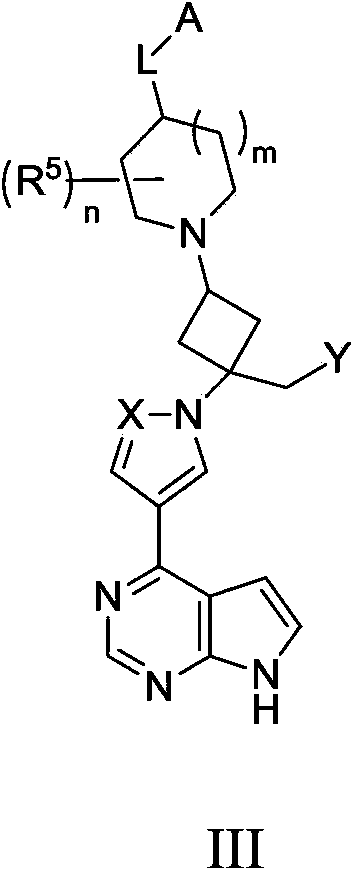
Cyclobutyl substituted pyrrolopyridine and pyrrolopyrimidine derivatives as JAK inhibitors
A cycloalkyl, heterocycloalkyl technology, applied in the field of cyclobutyl-substituted pyrrolopyridine and pyrrolopyrimidine derivatives as JAK inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0355] The preparation of the compounds of the present invention may involve the protection and deprotection of various chemical groups. One skilled in the art can readily determine the need for protection and deprotection and the choice of suitable protecting groups. Protecting group chemistry can be found, eg, in Wuts and Greene, Protective Groups in Organic Synthesis, 4th ed., John Wiley & Sons: New Jersey, (2007), which is hereby incorporated by reference in its entirety.
[0356] The reaction can be monitored according to any suitable method known in the art. For example, methods such as NMR spectroscopy (e.g., 1 H or 13 C), infrared spectroscopy, spectrophotometry (for example, UV-visible spectrophotometry), mass spectrometry, or by spectroscopic means such as high performance liquid chromatography (HPLC) or thin layer chromatography (thin layer chromatography; TLC) to monitor product formation.
[0357] Useful intermediates 3-4 can be prepared following the procedur...
Embodiment 1a
[0472] Example 1a. 3-[(4-{cis-3-(cyanomethyl)-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazole -1-yl]cyclobutyl}piperazin-1-yl)methyl]-5-fluorobenzonitrile
[0473]
[0474] Step 1. [2-Bromo-1-(bromomethyl)ethoxy](tert-butyl)diphenylsilane
[0475]
[0476] To a solution of 1,3-dibromo-2-propanol (20.00 g, 91.79 mmol) in dichloromethane (DCM) (100 mL) cooled to 0 °C was added 1H-imidazole (6.56 g, 96.4 mmol), Then tert-butylchlorodiphenylsilane (25.1 mL, 96.4 mmol) and 4-dimethylaminopyridine (1.12 g, 9.18 mmol) were added. The reaction was stirred while warming to room temperature overnight. The reaction mixture was diluted with ether, washed with water, and the aqueous layer was extracted once more with ether. The combined organic extracts were washed with water, then brine, dried over sodium sulfate, decanted and concentrated. Flash chromatography (eluting with a gradient of 0% ethyl acetate / hexane to 15% ethyl acetate / hexane) gave the desired product (42 g, 100...
Embodiment 1b
[0506] Example 1b. 3-[(4-{trans-3-(cyanomethyl)-3-[4-(7H-pyrrolo[2,3-d]pyrimidin 4-yl)-1H-pyrazole- 1-yl]cyclobutyl}piperazin-1-yl)methyl]-5-fluorobenzonitrile
[0507]
[0508] Step 1. {trans-3-piperazin-1-yl-1-[4-(7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2 ,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]cyclobutyl}acetonitrile
[0509]
[0510] To 4-{trans-3-(cyanomethyl)-3-[4-(7-{[2-(trimethylsilyl)ethoxy]methyl}-7H-pyrrolo[2, 3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]cyclobutyl}piperazine-1-carboxylic acid tert-butyl ester (1.58 g, 2.66 mmol, peak 2 from step 8 of Example 1a) To a solution in 1,4-dioxane (100 mL) was added 4.0 M aqueous hydrogen chloride (20 mL) and stirred overnight for two nights. The reaction mixture was poured into saturated sodium bicarbonate in an amount sufficient to neutralize and render the reaction mixture basic. Subsequently, dioxane was removed from the mixture in vacuo. The product was extracted with three portions of ethyl acetate. The comb...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com