Hydrogel transdermal patch for preventing congealing cold blood stasis-type primary dysmenorrheal, and preparation method of hydrogel transdermal patch
A technology for primary dysmenorrhea and transdermal patch, which is applied in the directions of non-central analgesics, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve the problem of low bioavailability and first-pass effect. Gastrointestinal tract damage and other problems, to achieve the effect of improving bioavailability, facilitating drug transdermal, and good anti-dysmenorrhea effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A transdermal patch with anti-cold blood stasis type primary dysmenorrhea effect, comprising a backing layer, a drug-containing matrix layer and an anti-adhesive layer, the preparation method of which comprises the following steps:
[0034] (1) Take 900g of angelica, 300g of Chuanxiong, 600g of red peony, 300g of cinnamon, 150g of cumin, 600g of Wulingzhi, 300g of myrrh, 900g of Puhuang, 300g of Corydalis, 300g of dried ginger, add 8 times the amount of water and soak for 12 hours, After decocting for 2 hours, pour out the decoction, continue to add 8 times the amount of water to decoct for 2 hours, collect the volatile oil parts with a volatile oil extractor during decoction, combine the two decoctions, and concentrate under reduced pressure at 50°C to obtain a concentration of 1 g Crude drug / ml concentrated solution 4600 ml, then add 95% ethanol to make the concentration of ethanol in the concentrated solution reach 80%, let it stand for 24 hours, take the supernatant ...
Embodiment 2
[0038] A transdermal patch with anti-cold blood stasis type primary dysmenorrhea effect, comprising a backing layer, a drug-containing matrix layer and an anti-adhesive layer, the preparation method of which comprises the following steps:
[0039] (1) Take 450g of angelica, 140g of Chuanxiong, 300g of red peony, 150g of cinnamon, 75g of cumin, 300g of Wulingzhi, 150g of myrrh, 450g of Puhuang, 150g of Corydalis, 150g of dried ginger, add 6 times the amount of water and soak for 10 hours, After decocting for 2 hours, pour out the decoction, continue to add 6 times the amount of water to decoct for 2 hours, use a volatile oil extractor to collect the volatile oil during decoction, combine two decoctions, and concentrate under reduced pressure at 50°C to obtain a concentration of 1 g 2300 ml of concentrated solution of crude drug / ml, then add ethanol with a concentration of 95% to make the concentration of ethanol in the concentrated solution reach 70%, let it stand for 24 hours, ...
Embodiment 3
[0042] Example 3 Blood circulation promoting and blood stasis removing test
[0043] 1 Experimental materials
[0044] 1.1 Experimental Animals SD female rats used in the experiment, weighing 200g±10g, were provided by the Experimental Animal Center (SPF level) of Nanjing Medical University (license number SCXK (Su) 2002-0031).
[0045] 1.2 Reagent Epinephrine hydrochloride solution, produced by Southwest Pharmaceutical Co., Ltd., batch number: 071101; sodium citrate, produced by Tianjin Biochemical Pharmaceutical Factory, batch number: 20071107
[0046] 1.3 Instrument LG-R-80 computerized blood viscosity tester, product of Beijing Shidi Scientific Instrument Company.
[0047] 2 Experimental methods
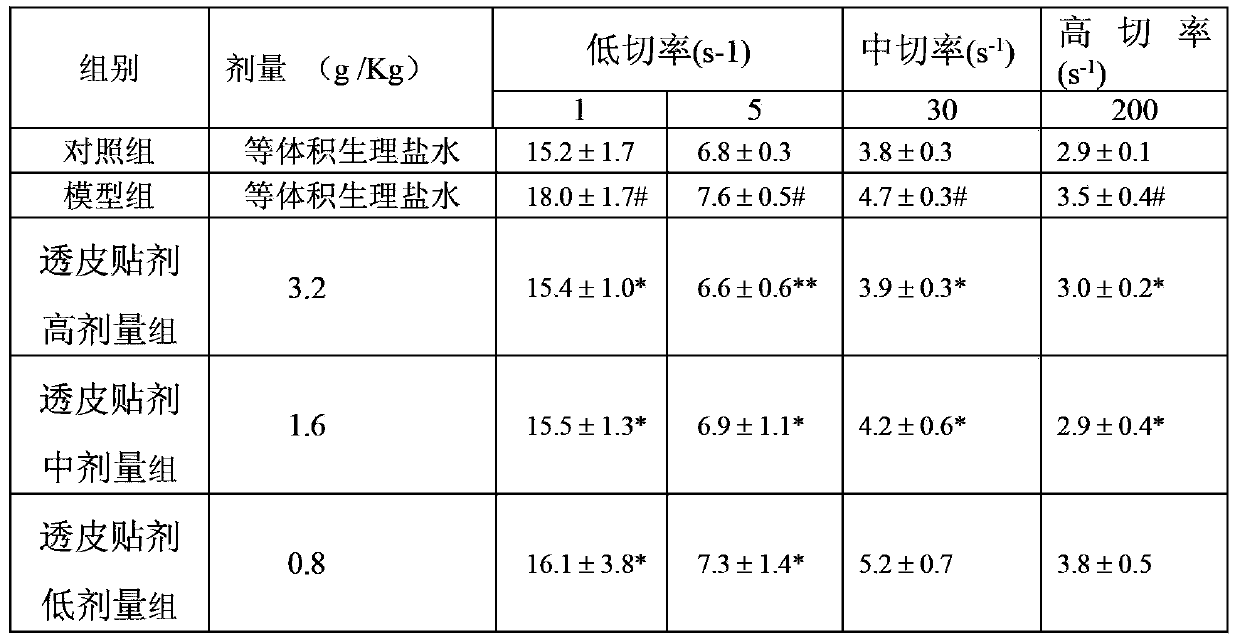
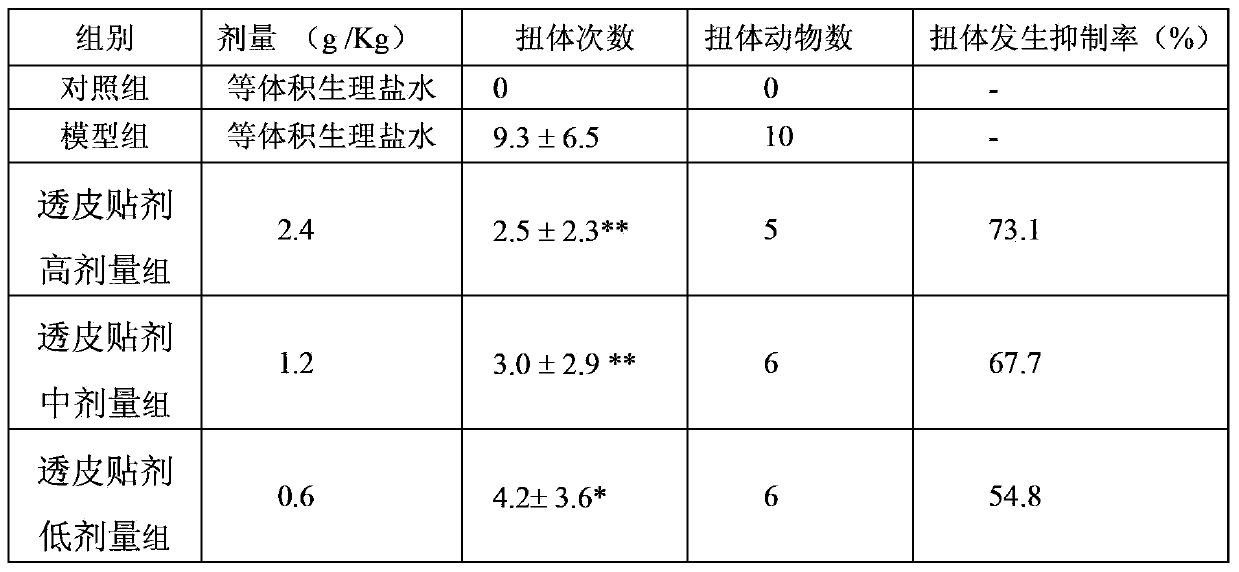
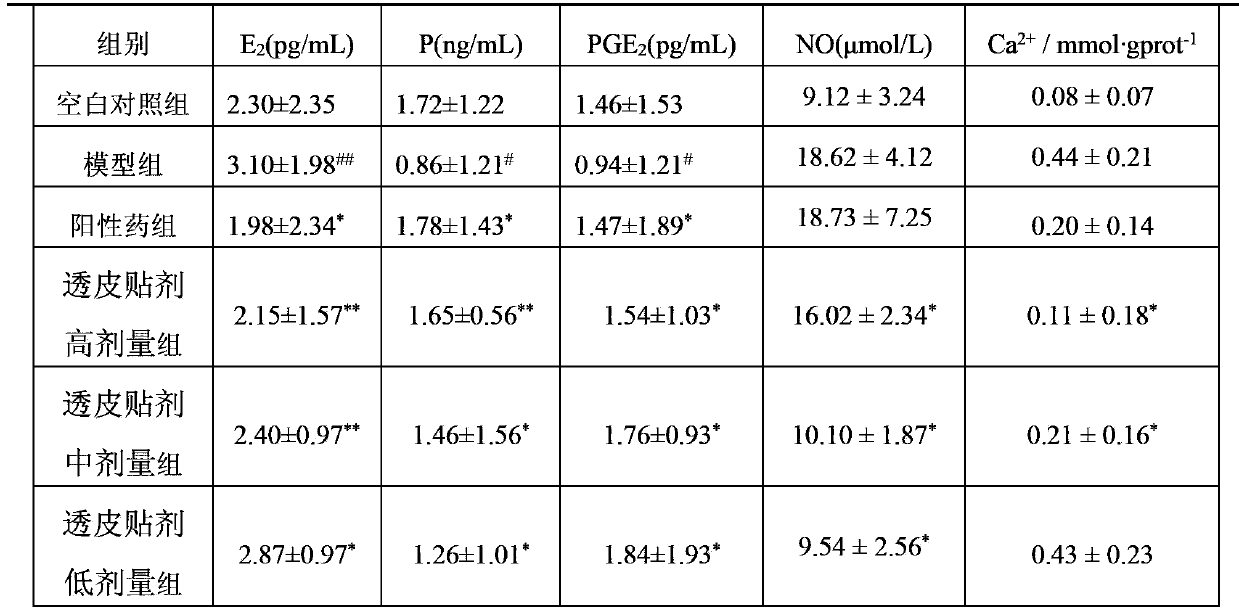
[0048] 2.1 Model preparation The normal group was fed routinely, and the rats in the model group and treatment group (attached with the patch prepared in Example 1 of the present invention) were placed in ice water at 0°C to 1°C for 5 minutes, once a day for seven consecutive d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com