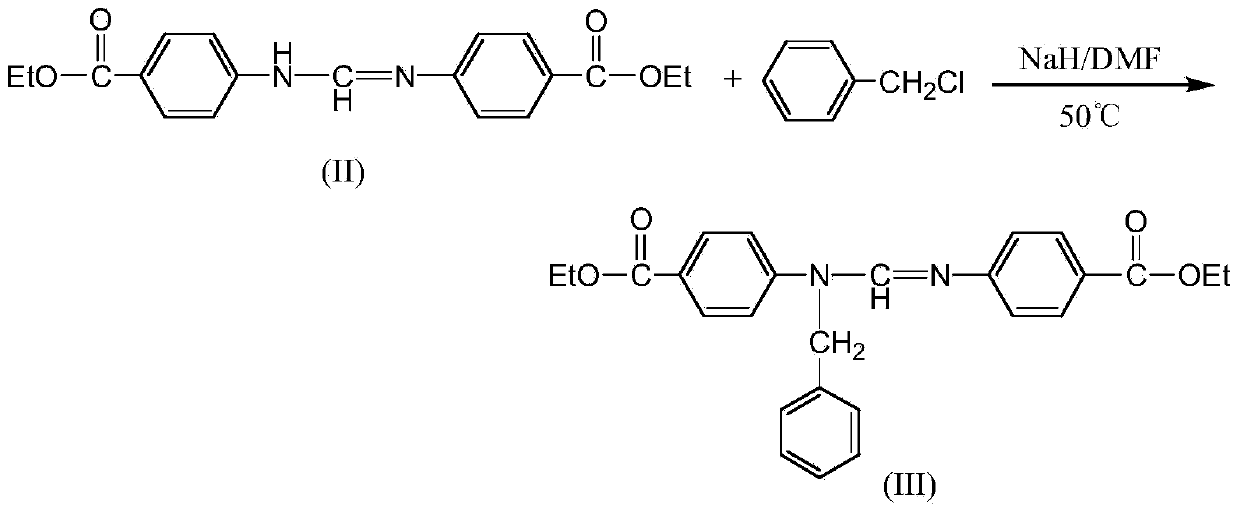
N, N'-di (4-ethyoxyl carbonyl phenyl)-N'-benzyl formamidine preparation method
A technology of ethoxycarbonyl phenyl and benzyl formamidine, which is applied in the field of preparation of benzyl formamidine compounds, can solve the problem that the product yield is not greatly improved, the N-alkylaniline route is long, and the industrial production is unfavorable. problems, to achieve the effect of improving the total yield, reducing by-products, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
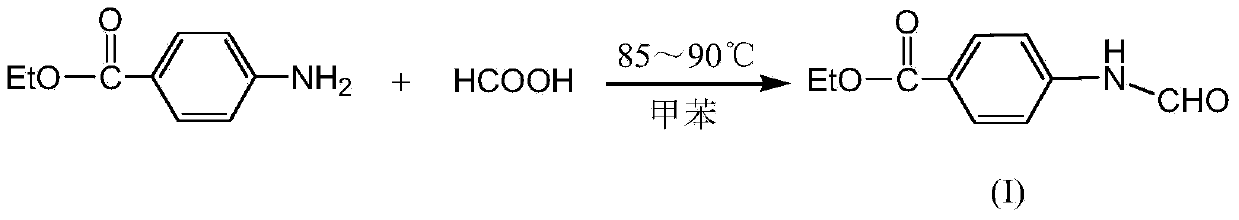
[0024] Example 1: Add ethyl p-aminobenzoate (49.5g, 0.3mol) and 450mL of toluene to a 1000mL four-necked reaction flask respectively, then slowly add formic acid (20g, 0.438mol) dropwise, and control the dropping within 0.5h. Raise the temperature to 85°C for 2 hours, then heat to about 105°C and distill at atmospheric pressure for about 1.5h. Stand still after cooling, precipitate a white solid, vacuum filtration, wash the solid with water several times until neutral, dry to obtain 57.32g of ethyl 4-formamidobenzoate (I), the yield is 99%.
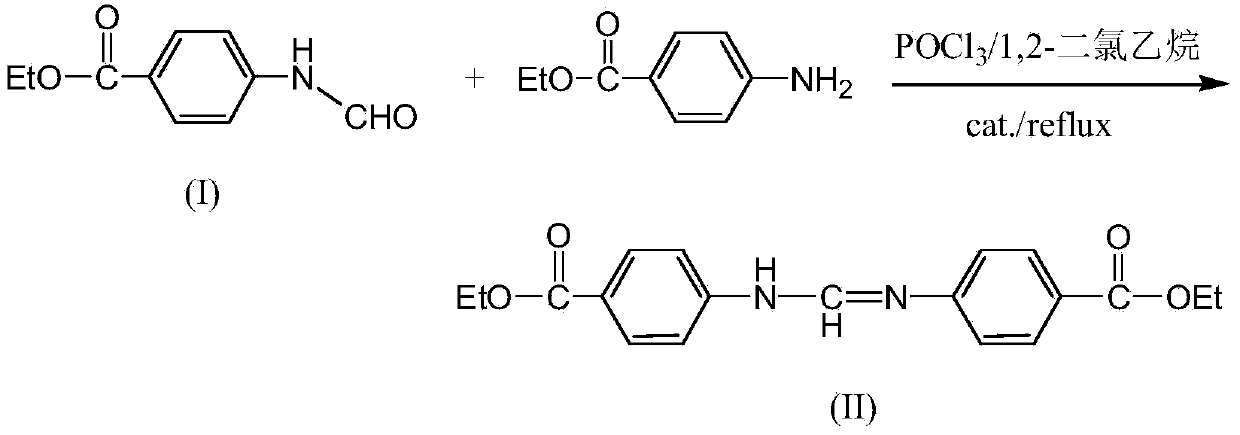
[0025] The above intermediate product (I) (39.2 g, 0.2 mol) was dissolved in 600 mL of 1,2-dichloroethane, and then POCl was added 3 (52g, 0.338mol), heated to 65°C to obtain a transparent solution, the reaction solution gradually became cloudy after 0.5h of reaction, continued to react for 0.5h, then added ethyl p-aminobenzoate (33g, 0.2mol) and tetrabutyl bromide Ammonium (0.05g), heated to reflux for 7 hours, cooled and poured into sa...
Embodiment 2
[0027] Example 2: Add ethyl p-aminobenzoate (49.5g, 0.3mol) and 450mL of toluene to a 1000mL four-necked reaction flask respectively, then slowly add formic acid (18.4g, 0.4mol) dropwise, and control the dropping within 0.5h , after heating up to 85°C for 2.5 hours, then heating to about 105°C for atmospheric distillation for about 1.5h. After cooling and standing, a white solid was precipitated, which was filtered under reduced pressure, washed with water several times until neutral, and dried to obtain 57.14 g of ethyl 4-formamidobenzoate (I), with a yield of 98.7%.
[0028] The above intermediate product (I) (39.2 g, 0.2 mol) was dissolved in 600 mL of 1,2-dichloroethane, and then POCl was added 3 (46g, 0.3mol), heated to 65°C to obtain a transparent solution, the reaction solution gradually became turbid after 0.5h of reaction, until the turbidity in the reaction solution did not deepen, then added ethyl p-aminobenzoate (33g, 0.2mol) and four Butylammonium bromide (0.06g)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com