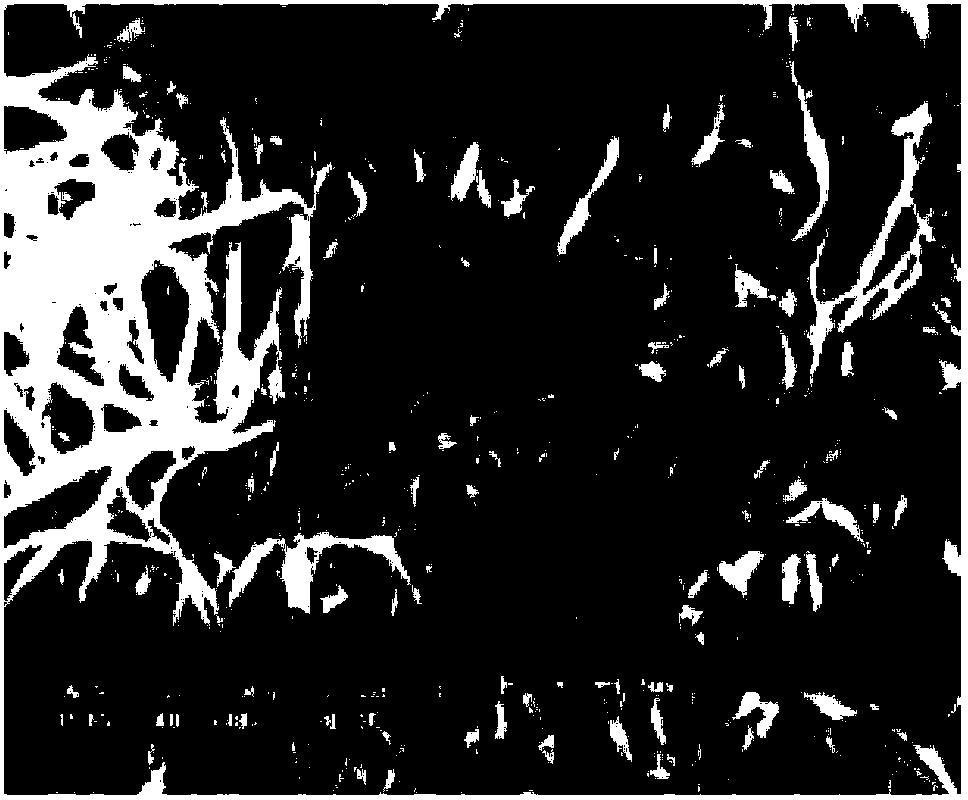
High-strength polyimide porous membrane including benzimidazole and benzene lateral groups and manufacturing method thereof
A technology of polyimide and benzimidazole, applied in the field of polyimide porous membrane and its preparation, can solve the problems of small interaction force between fibers, poor mechanical properties of porous membrane, small size, etc., to improve processing performance , Improve the mechanical strength, improve the effect of tensile strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A method for preparing a high-strength polyimide porous membrane includes the following steps:
[0040] 1. Synthesis of polyamic acid solution:
[0041] According to benzophenone tetraacid dianhydride (BTDA): 1,4-bis(4'-aminophenoxy)-2-(phenyl)benzene (TPEQ): 2-(4-aminophenyl)-5- Aminobenzimidazole (DAPBI) = 10:6:4 molar ratio for weighing and mixing, first add the two diamine monomers TPEQ and DAPBI with a molar ratio of 6:4 to the metered N,N-dimethyl In DMAc, make the total solid content 20%; then mechanically stir under the protection of nitrogen, and then add BTDA equal to the total amount of the two diamines in batches, and keep it under 25℃ in a nitrogen atmosphere The reaction was carried out for 5 hours to obtain a viscous polyamic acid solution. The polyamic acid solution was sealed and placed in a refrigerator for later use. After 24 hours, its viscosity was measured to be 5.6 Pa·s.
[0042] The structural formula of the polyamic acid polymer obtained in this syn...
Embodiment 2
[0049] 1. Synthesis of polyamic acid solution:
[0050] According to benzophenone tetraacid dianhydride (BTDA): 1,4-bis(4'-aminophenoxy)-2-(phenyl)benzene (TPEQ): 2-(4-aminophenyl)-5- Aminobenzimidazole (DAPBI) = 10:6:4 molar ratio for weighing and mixing, first add the two diamine monomers TPEQ and DAPBI with a molar ratio of 6:4 to the metered DMAc to make the total solid The content is 20%; then mechanically stirred under the protection of nitrogen, and then the BTDA equal to the total amount of the two diamines is added in batches, and the reaction is continued for 5 hours under a nitrogen atmosphere below 25°C to obtain viscous polyamic acid Solution. The polyamic acid solution was sealed and placed in a refrigerator for later use. After 24 hours, its viscosity was measured to be 5.6 Pa·s.
[0051] The structural formula of the polyamic acid polymer obtained in this synthesis step is as follows:
[0052]
[0053] Where n:m=4:6.
[0054] 2. Preparation of polyimide porous mem...
Embodiment 3
[0059] 1. Synthesis of polyamic acid solution:
[0060] According to benzophenone tetraacid dianhydride (BTDA): 1,4-bis(4'-aminophenoxy)-2-(phenyl)benzene (TPEQ): 2-(4-aminophenyl)-5- Aminobenzimidazole (DAPBI) = 10:6:4 molar ratio for weighing and mixing, first add the two diamine monomers TPEQ and DAPBI with a molar ratio of 6:4 to the metered DMAc to make the total solid The content is 20%; then mechanically stirred under the protection of nitrogen, and then the BTDA equal to the total amount of the two diamines is added in batches, and the reaction is continued for 5 hours under a nitrogen atmosphere below 25°C to obtain viscous polyamic acid Solution. The polyamic acid solution was sealed and placed in a refrigerator for later use. After 24 hours, its viscosity was measured to be 5.6 Pa·s.
[0061] The structural formula of the polyamic acid polymer obtained in this synthesis step is as follows:
[0062]
[0063] Where n:m=4:6.
[0064] 2. Preparation of polyimide porous mem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com