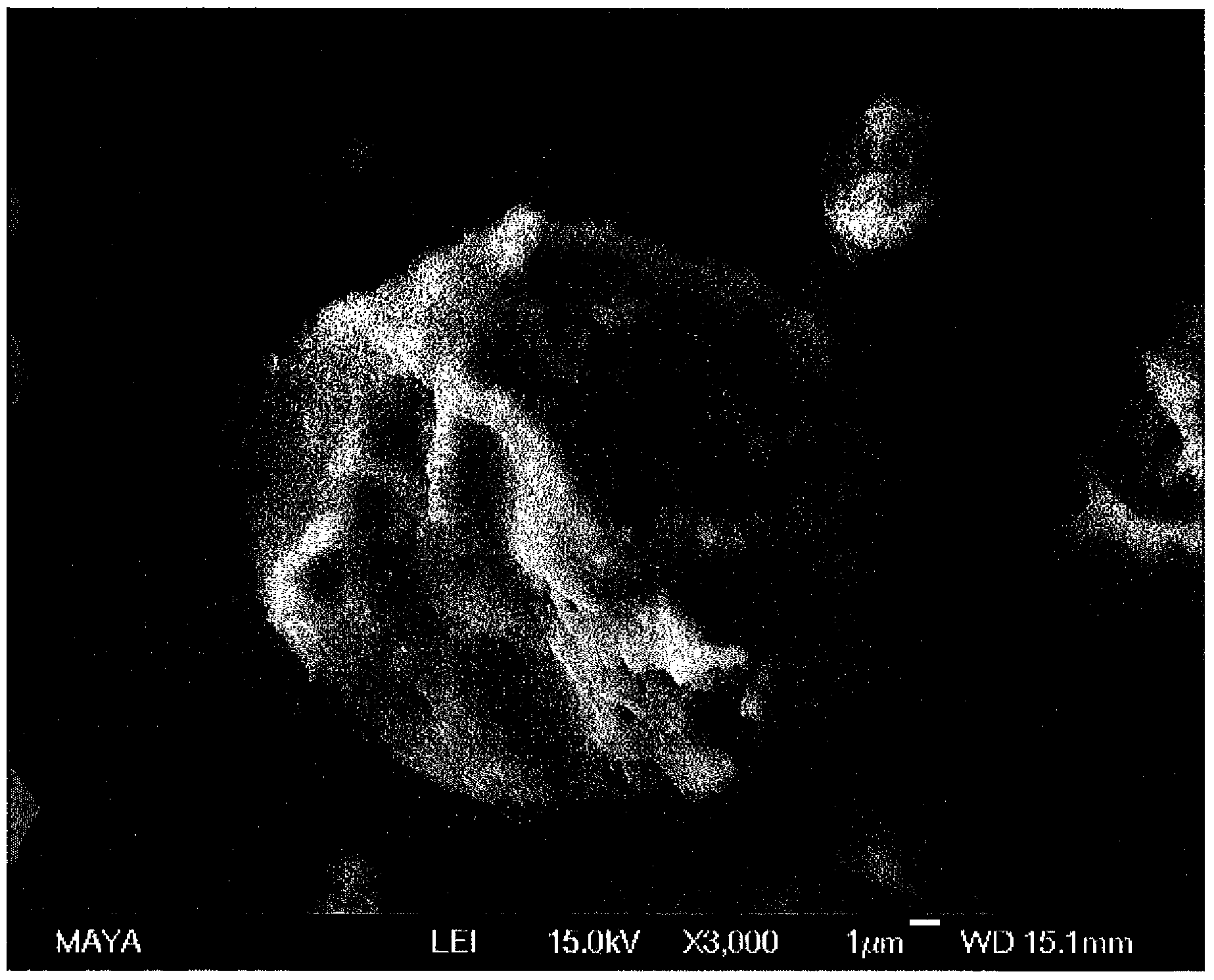
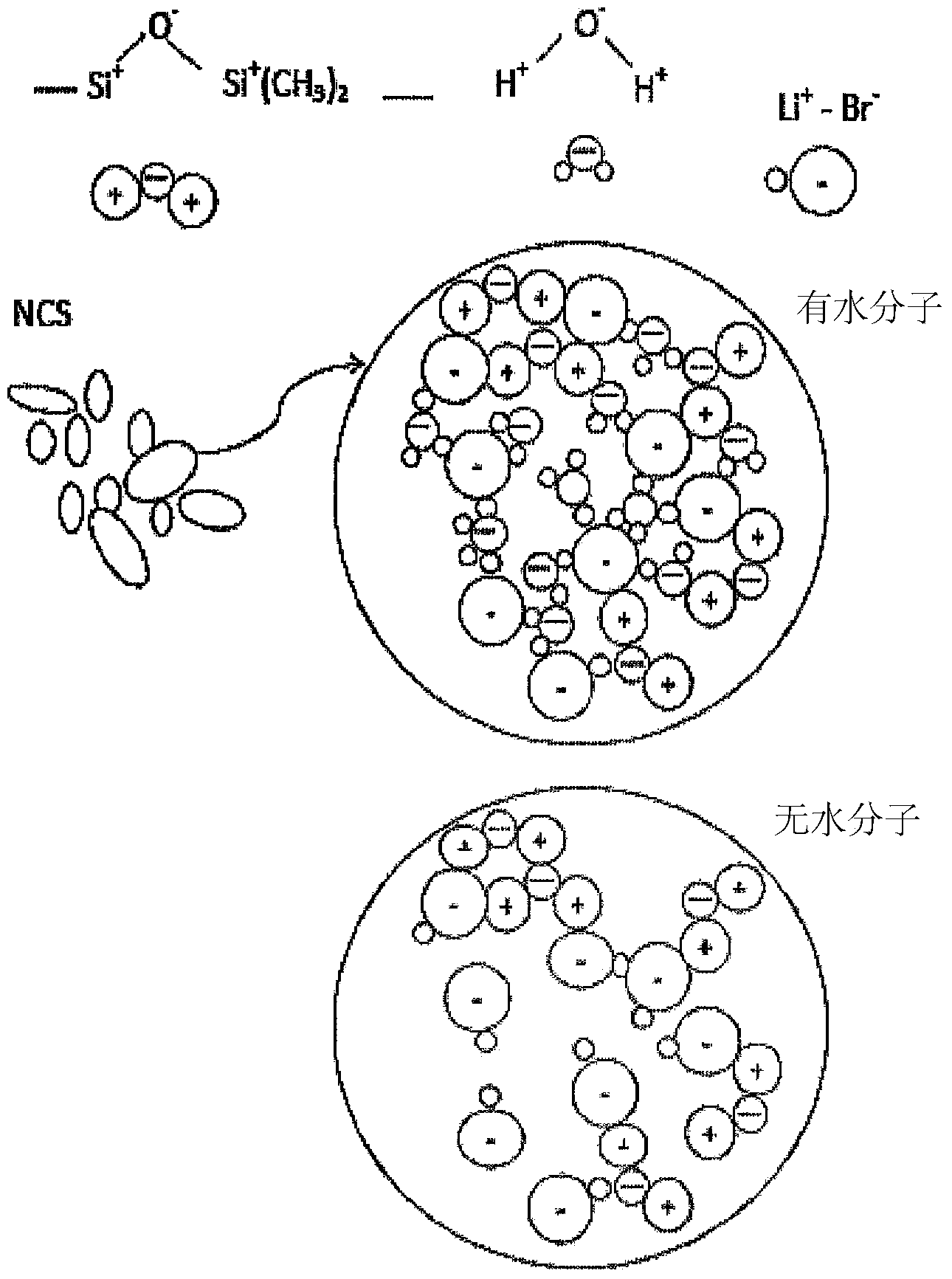
Salt coated with nanoparticles
A nanoparticle and particle technology, applied in the field of particles, can solve the problems of water evaporation, easy cracking, and it is difficult to obtain a fully reversible process, and achieve the effect of eliminating corrosion and improving long-term stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] In one experiment 95 parts of an aqueous solution of LiBr (32 wt%) was poured into an OBH Noordica type 1.5 L mixer and 5 parts of a hydrophobic silica derivative was added to the saline solution. Mixing was carried out at >10000 rpm in 3 time intervals, each time interval lasting about 30 s. The resulting material was a dry and free-flowing white powder. The nanoparticle-coated salt is then heat-treated.
Embodiment 2
[0094] Example 2 - Corrosivity to Copper, Steel and Aluminum
[0095] Nanoparticle-coated salt prepared according to Example 1 above. The original LiBr content in the aqueous solution was 32 wt%.
[0096] A spoonful of nanoparticle-coated salt was placed on three different metals:
[0097] copper
[0098] steel
[0099] aluminum
[0100] The metal was heated at 300°C for about 1 hour in an oven under atmospheric conditions.
[0101] For comparison, a 32 wt% lithium bromide aqueous solution was poured on a copper plate and heated on a hot plate (below 300°C) for about 15 minutes.
[0102] Corrosion quickly occurred on the copper sheet when using an aqueous salt solution. A blue / green oxidation product became very clear and formed a hole in the plate. The copper sheets exposed to the nanoparticle-coated salt did not show any signs of corrosion.
[0103] Metal sheets of steel and aluminum also did not show any signs of corrosion when exposed to the nanoparticle-coated s...
Embodiment 3
[0104] Example 3 - Reversibility of nanoparticle-coated salts when used in an absorption process
[0105] The nanoparticle coated salt was prepared according to Example 1 above. The original LiBr content in the aqueous solution was 32 wt%. 50 g of nanoparticle-coated salt was added to a small scale reactor, and the nanoparticle-coated salt contained 34 g of water. The reactor is connected to the condenser / evaporator via a gas delivery channel. Add 100 g of water to the condenser / evaporator.
[0106] The absorber was charged by heating the reactor to 120-150°C over 4-12 hours using cooling fins at about 6°C on the side of the condenser / evaporator.
[0107] The absorber was released by heating the condenser / evaporator with approximately 25-30°C cooling fins attached to the reactor to 17°C.
[0108] During the charging process, moisture evaporates from the nanoparticle-coated salt and is transported as water vapor to the condenser / evaporator where it condenses to form pure li...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com