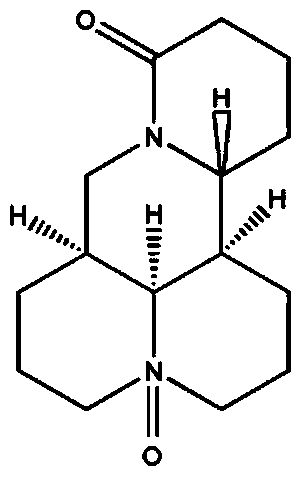
Application of oxymatrine in preparation of medicine for prevention and treatment of diabetic nephropathy
A technology for oxymatrine and diabetic nephropathy, applied in drug combination, urinary system diseases, metabolic diseases, etc., can solve problems such as poor targeting and inability to repair kidneys
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
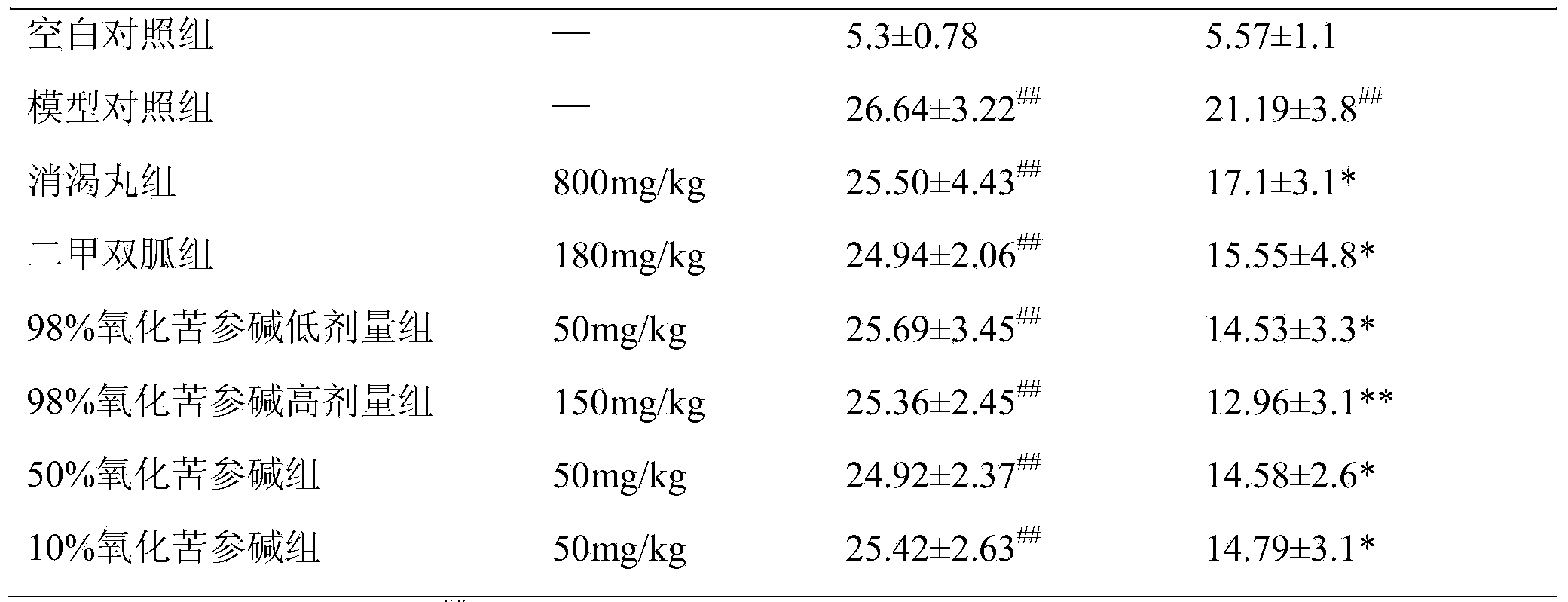
[0029] Example 1. Experimental study on the prevention and treatment of diabetic nephropathy by oxymatrine
[0030] 1. Modeling and drug administration
[0031] The animal model used is the combination of high-fat diet and STZ, and the resulting diabetic model rats have the characteristics of both type 1 and type 2 diabetes.
[0032] After 1 week of adaptive feeding, 8 rats were randomly selected as blank control group, and the group was fed with normal feed; the remaining rats were fed with high-sugar and high-fat feed (70% basal feed, 15% sucrose, 10% lard). and 5% egg yolk powder). After 4 weeks of continuous feeding, rats fed a high-sugar and high-fat diet were given a one-time intraperitoneal injection of 5 mL / kg of newly prepared STZ solution with a concentration of 6 mg / mL, and rats in the blank control group were given a one-time intraperitoneal injection of citric acid-sodium citrate buffer. Salt solution 5mL / kg. After 1 week of intraperitoneal injection, the rats ...
Embodiment 2 8
[0086] Example 2. Sample preparations of 98% oxymatrine, 50% oxymatrine and 10% oxymatrine
[0087] The preparation method of 98% oxymatrine capsules: take the 98.2% oxymatrine powder in Example 1 with a purity of 98.2%, add 1%-15% starch or other pharmaceutically acceptable carriers or conventional edible excipients (or other pharmaceutically acceptable Acceptable carrier or conventional food adjuvant: pharmaceutically acceptable carrier includes oral preparation adjuvant or parenteral administration adjuvant. The route of administration can be oral, injection, local administration, etc. According to the technical scheme of the present invention, The composition can be an oral preparation or an injection preparation, wherein the oral preparation includes capsules, soft capsules, granules, oral liquids, tablets, dropping pills, etc. The auxiliary materials used include: starch, sucrose, lactose, powdered sugar, glucose , Mannitol, Xylitol, Polyethylene Glycol, Isopropyl Alcohol,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com