Method for researching abirritation mechanism of Chinese herbal medicinal ingredients of Xinhuang tablets
A technology of Xinhuang tablets, anti-inflammatory and analgesic drugs, applied in the field of traditional Chinese medicine, can solve the problem of insufficient analgesic effect for severe pain, and achieve good drug safety, good synergistic effect, and excellent therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
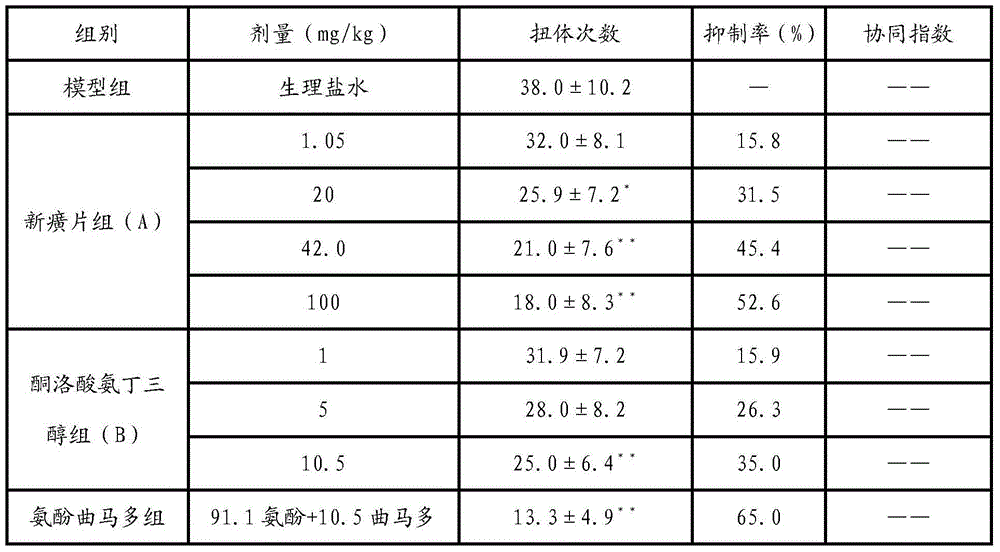
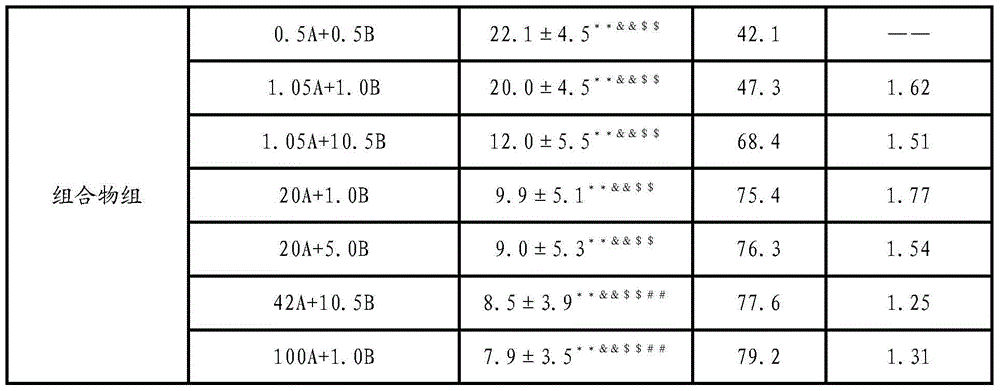
[0035] The composition of embodiment 1 Xin Huang tablet and ketorolac trometamol is to the influence of the mice writhing number of times that acetic acid causes
[0036] 1. Grouping and administration of animals
[0037] Seventy male ICR mice, (20±2) g, were randomly divided into groups according to body weight, 10 in each group, and each group was given therapeutic drugs by intragastric administration. Xinhuang Tablets, Ketorolac Tromethamine Tablets, and Tramadol Amphenol Tablets were respectively prepared into suspensions with normal saline, and then each administration group was administered intragastrically according to the dose setting; the model group was intragastrically administered equal volume of saline.
[0038] 2. Experimental methods and data processing
[0039] Intraperitoneal injection of acetic acid in mice caused large-scale and long-lasting pain stimulation in the deep abdominal cavity, resulting in writhing reactions in mice. After each dose group was a...
Embodiment 2
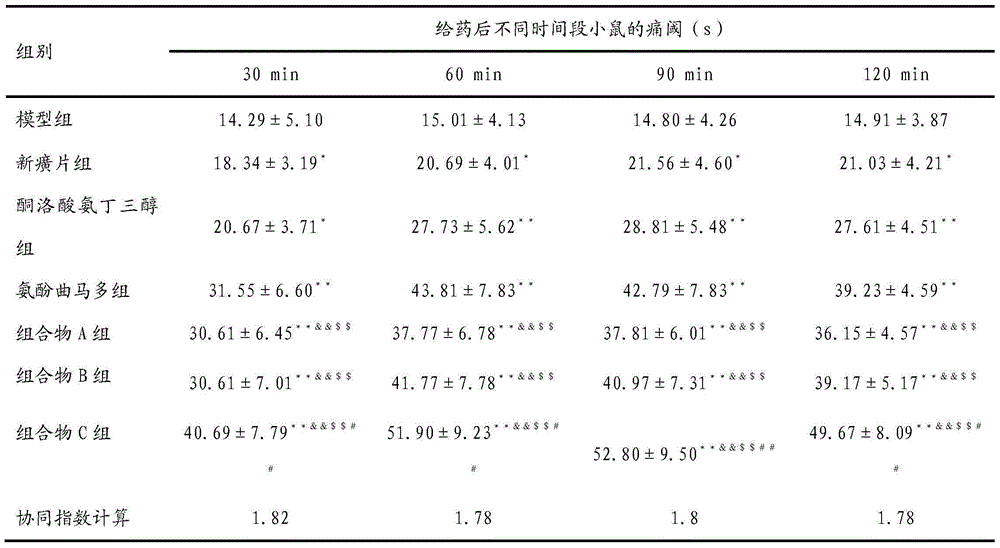
[0055] Effect of the composition of embodiment 2 Xin Huang tablet and ketorolac tromethamine on mouse hot plate pain response
[0056] 1. Grouping and administration of animals
[0057] Put female ICR mice (20±2) g on an intelligent hot plate instrument at 55±0.5°C, record the latency (s) from when the sole of the mouse touches the hot plate to the reaction of hind paw licking is recorded as the pain threshold index, and the reaction is eliminated Mice with latency 30s or jumping. Select 140 qualified mice with response latency within 10-30s, randomly group them into groups according to predrug pain threshold and body weight, and administer them by intragastric administration;
[0058] Model group: equal volume of normal saline, ig
[0059] Xinhuang tablet group: Xinhuang tablet 42.0mg / kg, ig
[0060] Ketorolac tromethamine: 10.5mg / kg, ig
[0061] Aminophen tramadol group: acetaminophen 91.1mg / kg + tramadol hydrochloride 10.5mg / kg, ig
[0062] Composition A group: Xinhuan...
Embodiment 3
[0082] Embodiment 3 The clinical therapeutic effect of the pharmaceutical composition of the present invention on cancer pain
[0083] 3.1 General information: Select 500 cancer pain patients from the First People's Hospital of Hebei Province between January 2009 and January 2010, aged 16-65 years, weighing more than 50kg, with an average age of 38.7 years and an average weight of 62.5kg . The diagnosis was confirmed by auxiliary examination, and no analgesic treatment was performed before seeing a doctor, and there were no complications such as pancreatitis and massive bleeding.
[0084] 3.2 Treatment method 500 patients were randomly divided into 4 groups, 125 cases in each group. Each group took medicine in the following way: Xinhuang Tablets group: take Xinhuang Tablets orally: take 3 Xinhuang Tablets orally (0.32g / tablet, produced by Xiamen Traditional Chinese Medicine Factory Co., Ltd.), three times a day; Alcohol group: take 20 mg of ketorolac tromethamine three times...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com