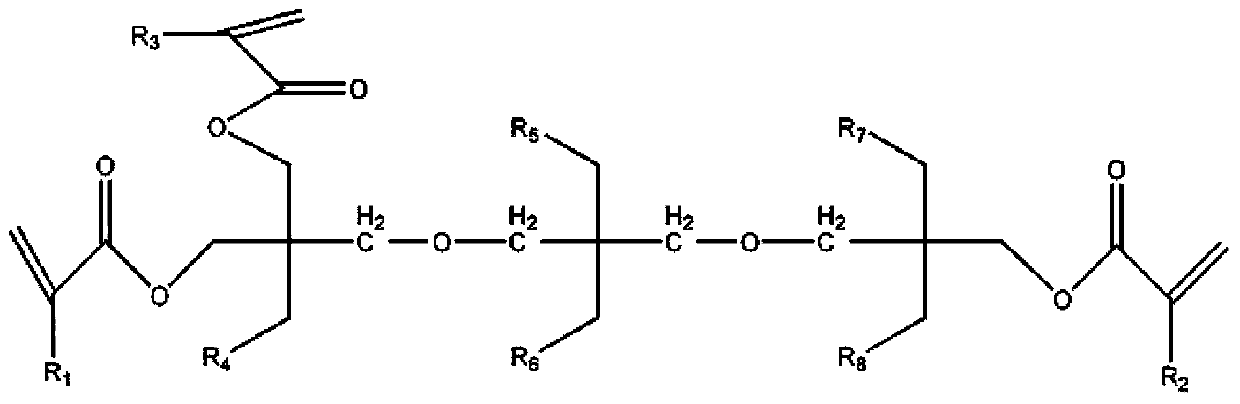
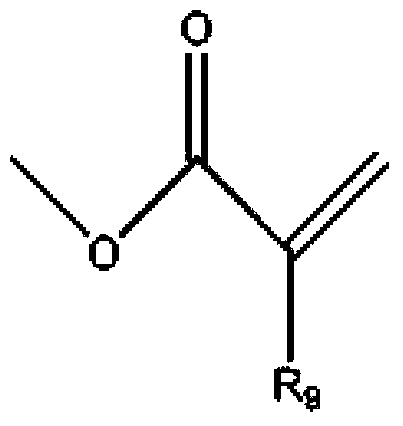
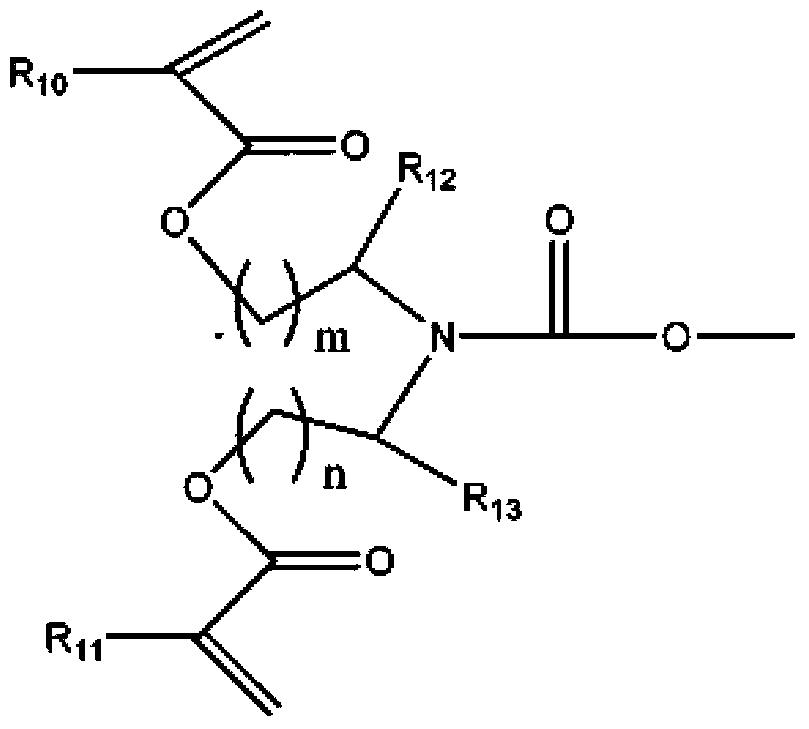
Modified tripentaerythritol acrylic ester with low viscosity, high reactivity and shrunk volume as well as preparation method thereof
A high-reactivity, tripentaerythritol technology, used in the preparation of organic compounds, the preparation of carbamic acid derivatives, chemical instruments and methods, etc., can solve the problems of hard cured film, poor dilution, high volume shrinkage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation of cyclic 1,3 propanediol carbonate-type modified tripentaerythritol from tripentaerythritol and dimethyl carbonate:
[0058] Add (186 g, 0.5 mol) tripentaerythritol, (180 g, 3 mol) dimethyl carbonate and (0.56 g, 5 mmol) 1,4-diazabicyclo[2.2.2]octane DABCO into a In a 2000ml reaction flask with a reflux condenser, place the flask in a sand bath in an electric heating mantle, use a temperature controller connected to a thermocouple immersed in the sand bath to control the temperature of the sand bath, use the sand bath to heat the contents of the solution in the flask with stirring To 80 ℃, by stirring, the solid component of the solution substance in the flask dissolves into a colorless transparent solution, the reaction temperature is maintained at 80 ℃ and continues to stir for 16 hours, the reaction product methanol is continuously distilled out during the reaction, and the excess dicarbonate The methyl ester was distilled out of the reaction bottle, and...
Embodiment 2
[0060] The cyclic 1,3 propanediol carbonate type modified tripentaerythritol and the diethanolamine reaction synthesis band tertiary amine modified tripentaerythritol polyol by embodiment 1:
[0061] Before cooling the reaction solution obtained in the flask of Example 1 to room temperature, (210g, 2mol) diethanolamine and 600ml cyclohexane were added, and under the action of electric stirring, the solution in the flask was slowly heated to 70°C, and the reaction temperature was maintained at 70°C. Continue to stir at ℃ for 3 hours, cool to room temperature after the reaction is completed, and perform multiple experiments at the same time under the same conditions as above. One of the obtained products is purified by ethanol and water precipitation, recrystallization, etc., and the reaction process and the end When the product solution is subjected to point plate analysis, it is separated by silica gel column chromatography (n-hexane: ethyl acetate = 4:1), and the rest of the o...
Embodiment 3
[0063] The cyclic 1,3 propanediol carbonate type modified tripentaerythritol and the diisopropanolamine reaction synthesis band tertiary amine modified tripentaerythritol polyol by embodiment 1:
[0064]Before cooling the reaction solution obtained in the flask of Example 1 to room temperature, (266g, 2mol) diisopropanolamine and 600ml cyclohexane were added, and under the action of electric stirring, the solution in the flask was slowly warmed up to 70°C. Before the sample was spotted on a silica gel plate and the reaction process and the product solution at the end were spot plate analyzed, the reaction temperature was maintained at 70 ° C and continued to stir for 3 hours, and cooled to room temperature after the reaction was completed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com