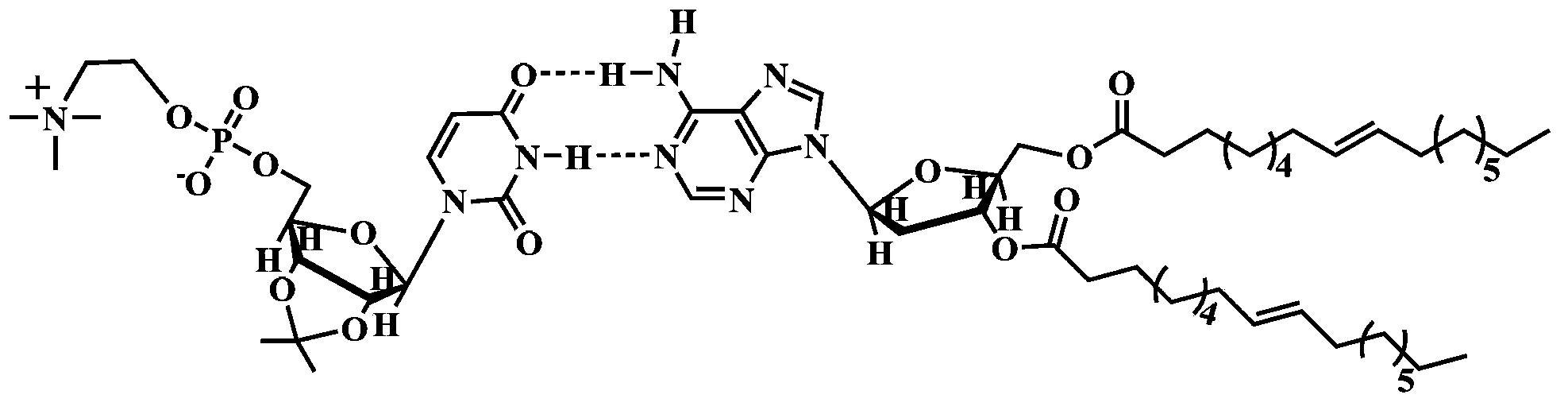
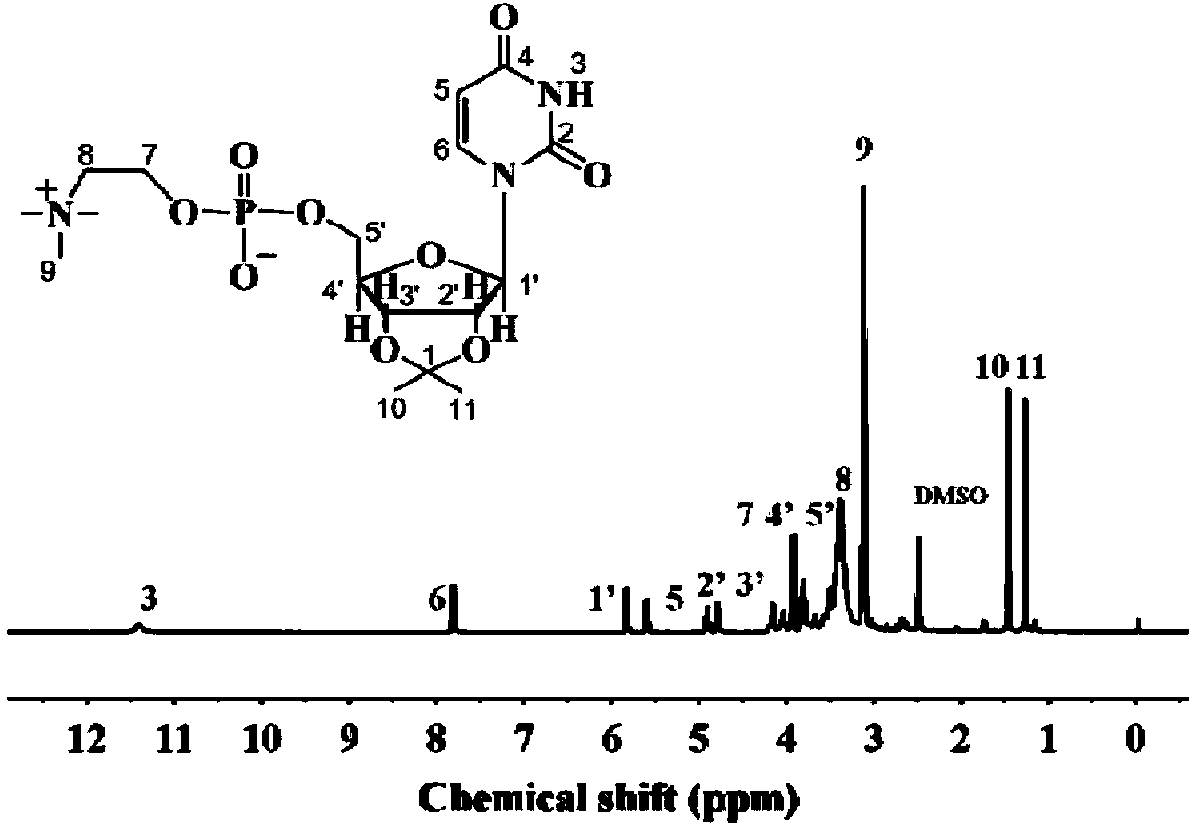
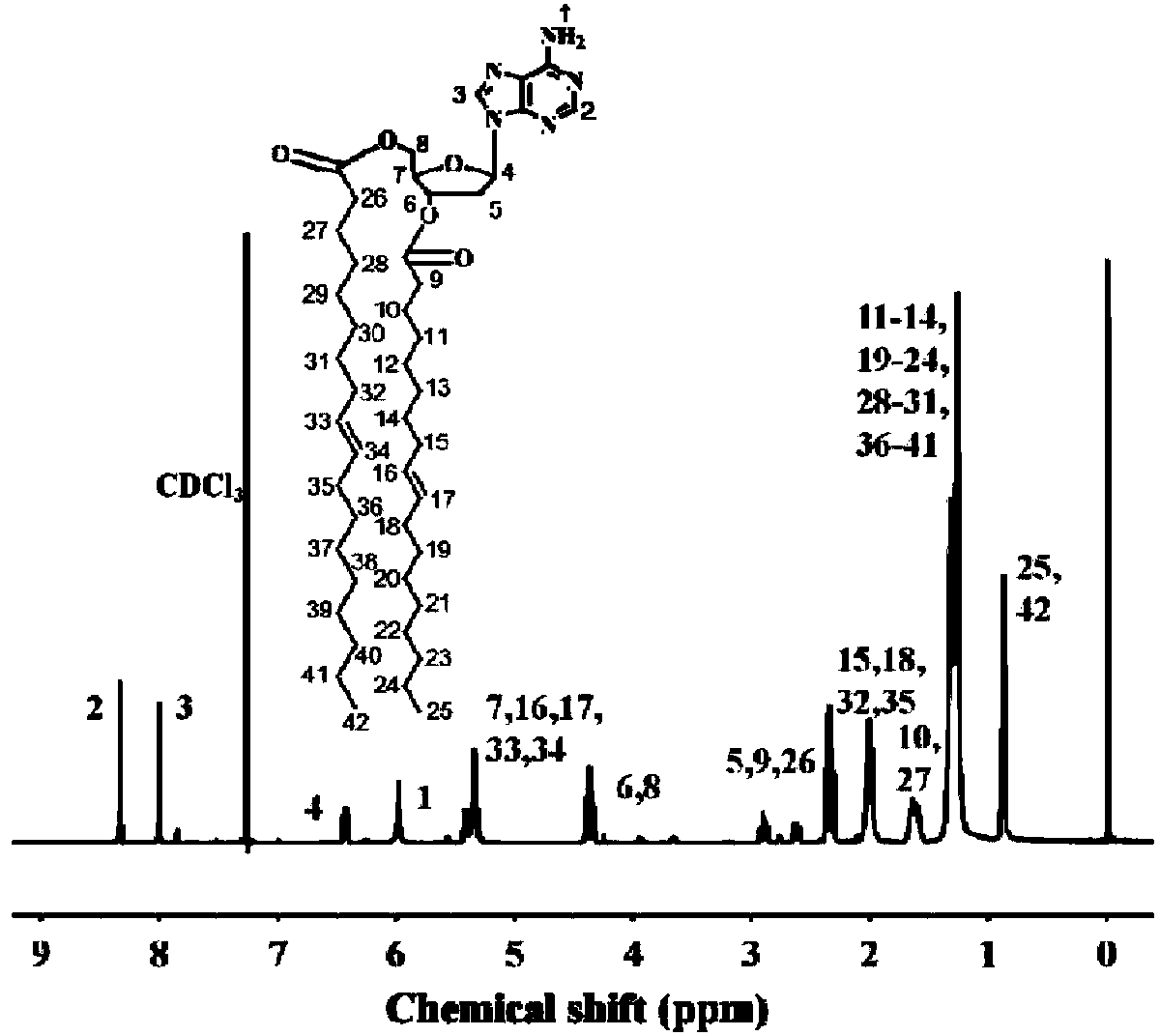
Supermolecule phospholipid based on nucleic acid bases, preparation method of supermolecule phospholipid and liposome
A nucleic acid base and molecular phospholipid technology, applied in the field of phospholipids, can solve problems such as hidden dangers of biocompatibility, poor acid responsiveness, complex synthesis, etc., and achieve excellent base biocompatibility, high stability, and simplified synthesis procedures Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Step a: Add 1.66 grams of trimethylamine and 22 milliliters of tetrahydrofuran solution to a 100 milliliter reaction flask, then add 1.08 grams of uridine modified by chlorophosphate vinyl ester, then add 10 milliliters of acetonitrile, and stir at 40 degrees Celsius for 48 hours , natural cooling to obtain light yellow solid - phosphorylcholine containing uridine.
[0046] Step b: Mix 0.5 g of adenosine, 2.25 g of oleic acid, 0.86 g of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride and 0.27 g of 4-dimethylaminopyridine (DMAP) Added to the reaction bottle, and then added 25 ml of chloroform, stirred at room temperature for 12 hours. The precipitated insoluble matter was removed by filtration, and the filtrate was separated by column chromatography with a mixture of dichloromethane and methanol (20:1) by volume as the eluent to obtain dioleoyl adenosine with a yield of 60%.
[0047]Step c: Dissolve the above-mentioned uridine phosphorylcholine and dioleoyl ad...
Embodiment 2
[0051] Step a: Add 0.95 grams of ammonia and 100 milliliters of tetrahydrofuran solution to a 200 milliliter reaction flask, then add 1.08 grams of chlorophosphate-modified uridine, then add 10 milliliters of acetonitrile, and stir at 80 degrees Celsius for 24 hours, After natural cooling, a light yellow solid-phosphoethanolamine containing uridine was obtained.
[0052] Step b: Mix 0.5 g of adenosine, 1.12 g of oleic acid, 0.86 g of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride and 0.27 g of 4-dimethylaminopyridine (DMAP) Add it into the reaction bottle, then add 25 ml of chloroform, and stir at room temperature for 24 hours. The precipitated insoluble matter was removed by filtration, and the filtrate was separated by column chromatography with a mixture of dichloromethane and methanol (20:1) by volume as the eluent to obtain dioleoyl adenosine with a yield of 60%.
[0053] Step c: Dissolve the above-mentioned uridine phosphoethanolamine and dioleoyl adenosine i...
Embodiment 3
[0056] Step a: Add 2.6 grams of trimethylamine and 22 milliliters of tetrahydrofuran solution in a 100 milliliter reaction flask, then add 1.08 grams of uridine modified by chlorophosphate vinyl ester, then add 10 milliliters of acetonitrile, and stir at 60 degrees Celsius for 36 hours , natural cooling to obtain light yellow solid - phosphorylcholine containing uridine.
[0057] Step b: Add 0.5 g of adenosine, 1.02 g of myristic acid, 0.86 g of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride and 0.27 g of 4-dimethylaminopyridine In the reaction flask, 25 ml of chloroform was added, and the reaction was stirred at room temperature for 24 hours. The precipitated insoluble matter was removed by filtration, and the filtrate was separated by column chromatography using a mixture of dichloromethane and methanol in a volume ratio of (20:1) as the eluent to obtain dimyristoyl adenosine with a yield of 67%.
[0058] Step c: Dissolve the above-mentioned uridine phosphorylcho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com