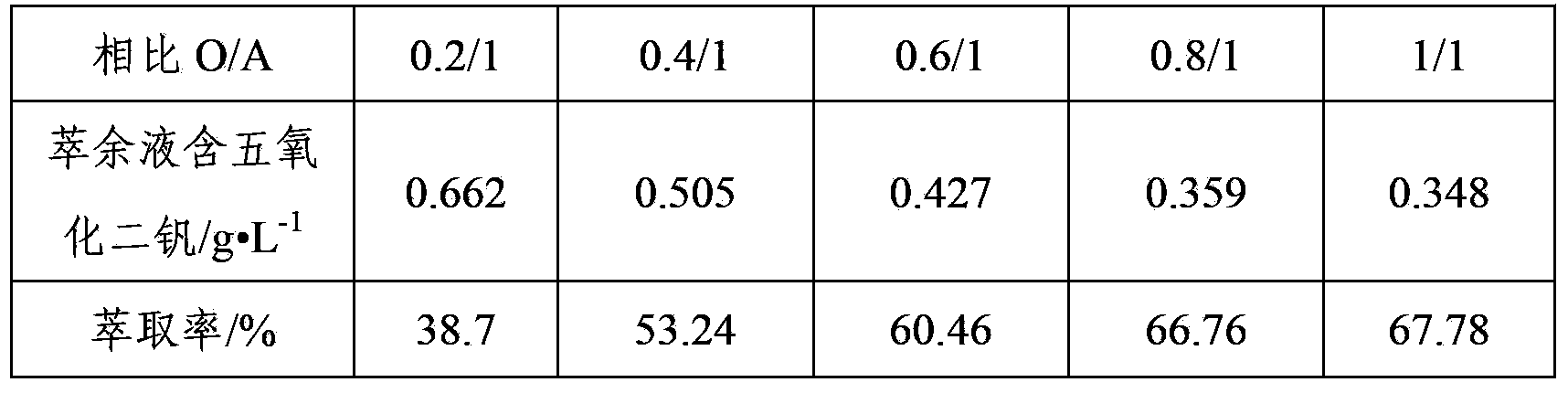
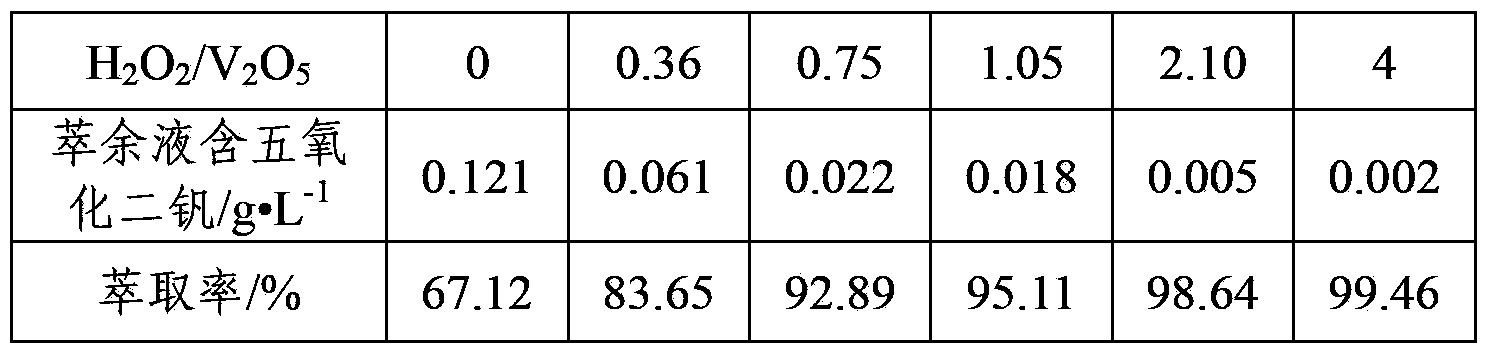
Method of extracting vanadium from stone coal
A stone coal and extraction technology, applied in the field of metallurgy and chemical industry, can solve the problems of complex process, large consumption of reagents, high cost, etc., and achieve the effect of simple process, easy operation and reduced production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
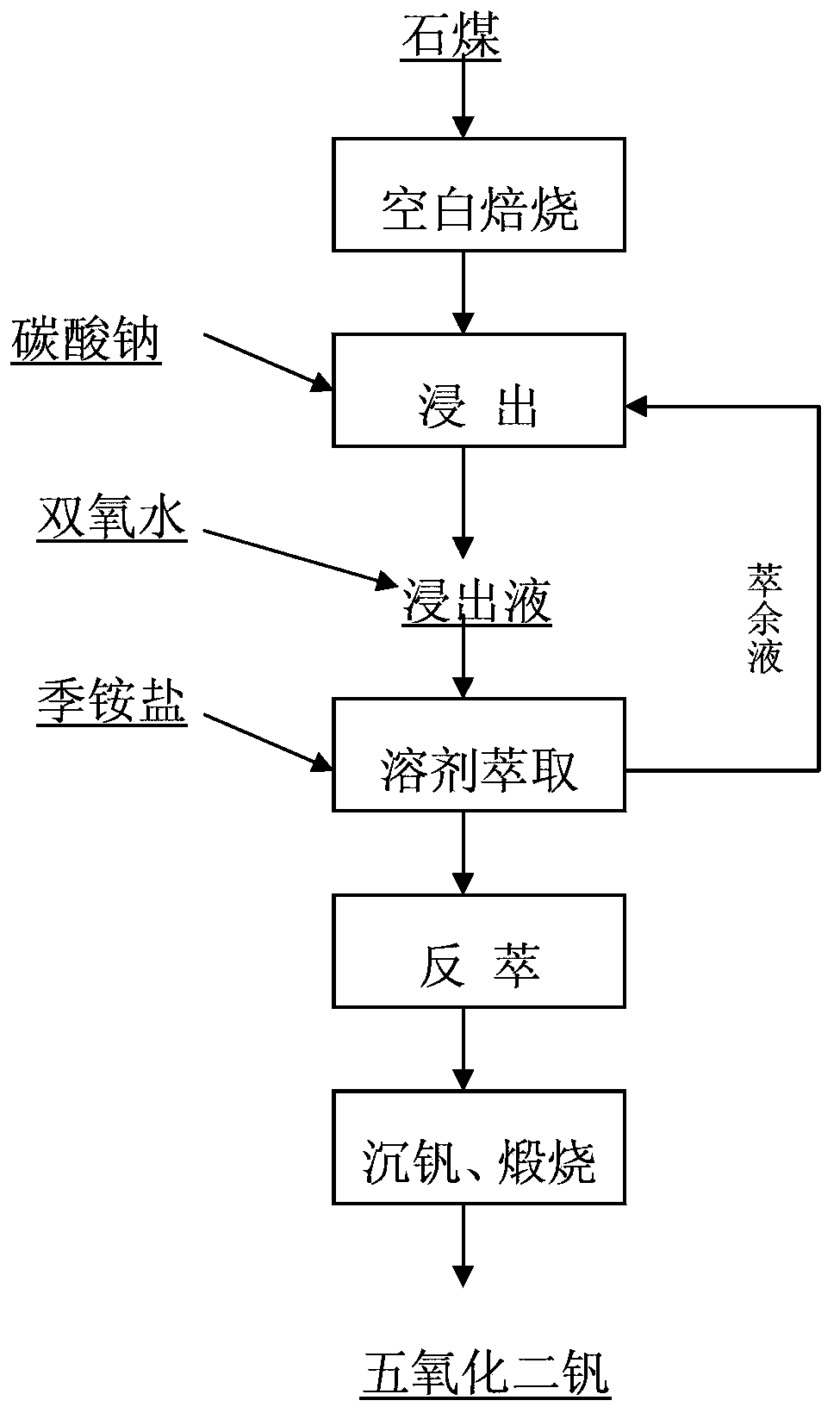
[0036] according to figure 1 The process flow is operated.
[0037] The content of vanadium pentoxide in stone coal roasting material is 1.2806%;
[0038] The leaching agent is anhydrous sodium carbonate, which is prepared into a 3% solution;
[0039] Conditions: liquid-solid ratio 1 / 1, time 2h, normal pressure, the amount of sodium carbonate added is 3% of the mass of stone coal roasting material (100 grams of calcined sand is added with 3 grams of sodium carbonate);
[0040] First, add anhydrous sodium carbonate to pure water at room temperature, stir to dissolve, heat to different temperatures in a water bath, and then add stone coal roasting material to it for leaching. The results are shown in Table 1.
[0041] The experimental results of leaching under different temperature conditions in table 1
[0042] temperature / ℃
[0043] The results showed that more than 50% of vanadium was leached at 10-90°C.
Embodiment 2
[0045] Raw material is with embodiment 1.
[0046] The leaching agent is anhydrous sodium carbonate (analytical pure);
[0047] Conditions: liquid-solid ratio 1 / 1, time 1h, normal pressure, temperature 80°C;
[0048] First, add anhydrous sodium carbonate to pure water at room temperature to make different concentrations (the first line of Table 2), stir to dissolve, heat in a water bath at 80°C, and then add stone coal roasting material to it for leaching. The results are shown in the table 2.
[0049] Table 2 Leaching test results under different leaching agents
[0050] Leaching agent / %
Embodiment 3
[0052] Raw material is with embodiment 1. The leaching agent is sodium carbonate solution;
[0053] Conditions: liquid-solid ratio 1 / 1, time 1h, normal pressure, temperature 80°C;
[0054] First, add anhydrous sodium carbonate to pure water at room temperature, stir and dissolve into a 10% aqueous solution, heat it in a water bath at 80°C, and then add stone coal roasting material to it for leaching. The vanadium leaching rate is 60.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com