Copolymer blue light optical material, preparation method, and applications thereof
A blue-light luminescence and copolymer technology, which is applied in luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve the problem of light emission color stability, low device luminous efficiency, and high hole injection that affect the saturation color purity of the device. and other problems, to achieve the effect of improving solubility and film-forming properties, good solubility, and reduced manufacturing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
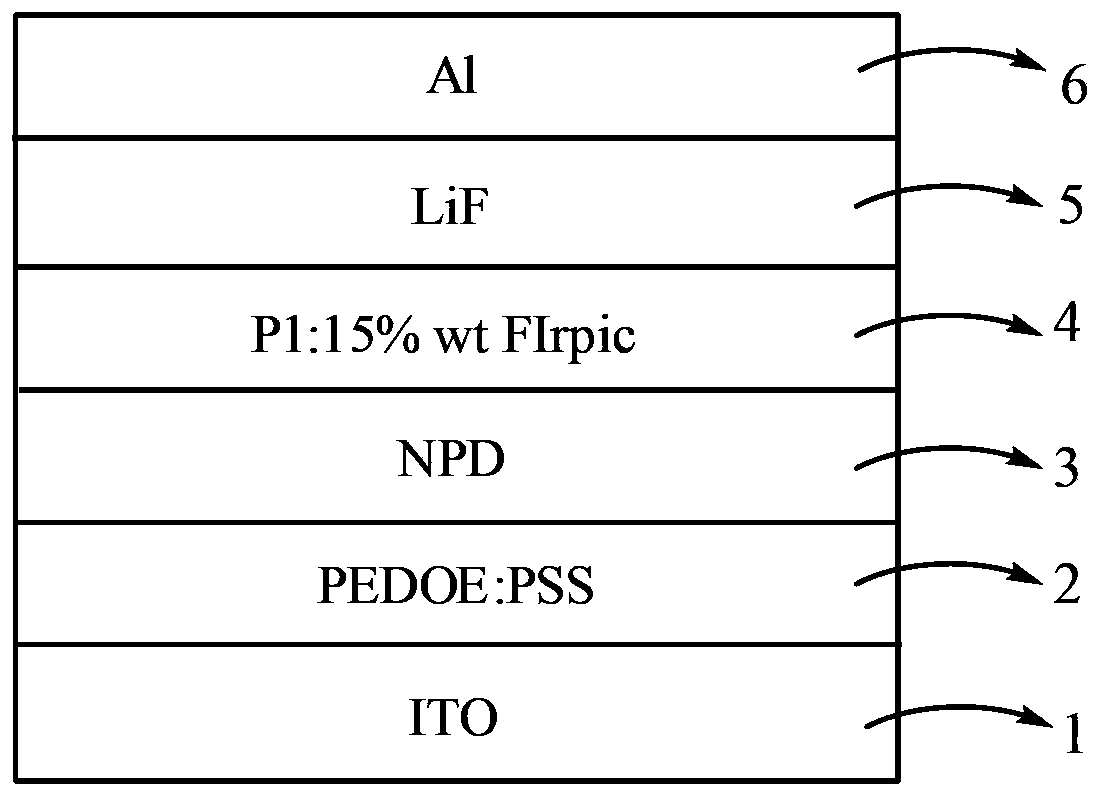
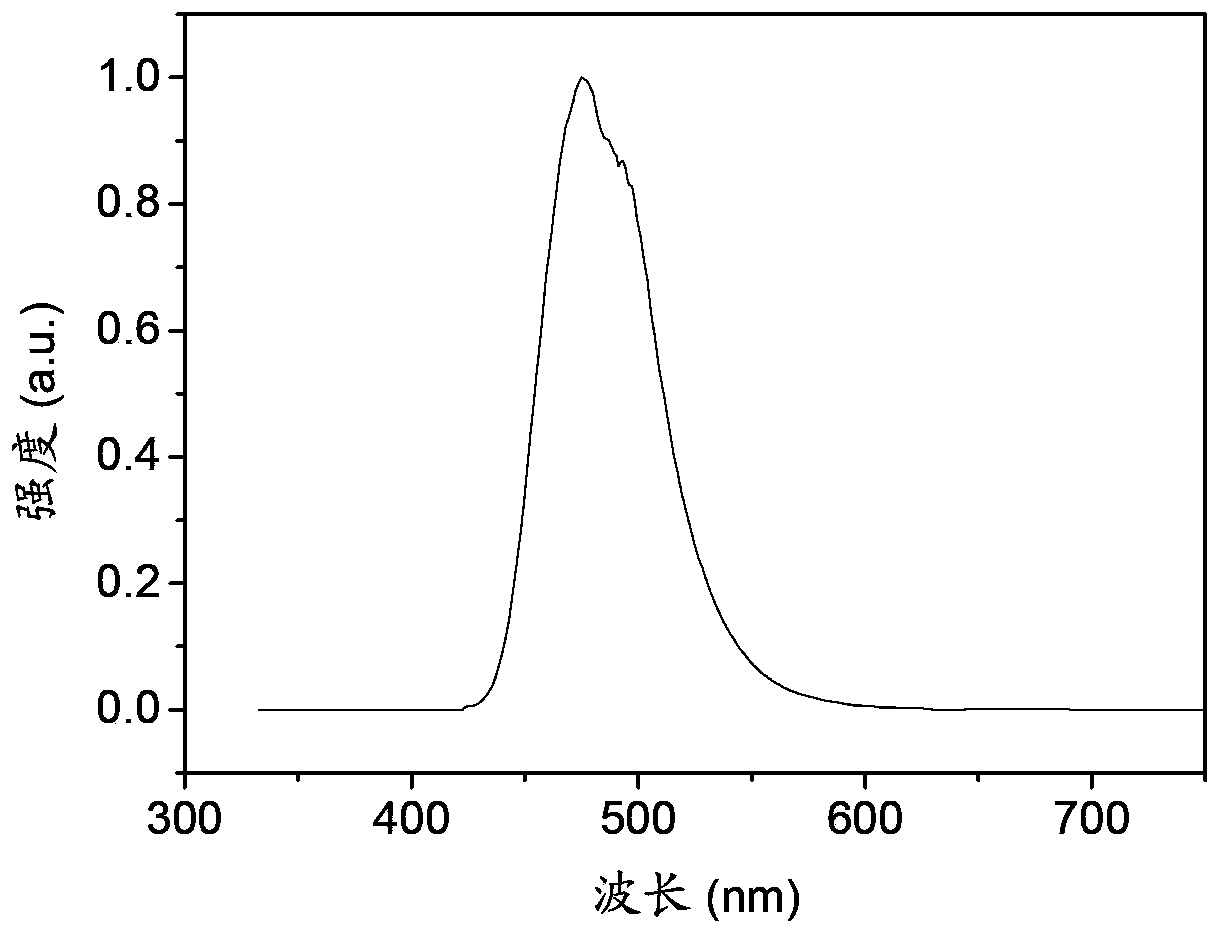
Image
Examples
preparation example Construction
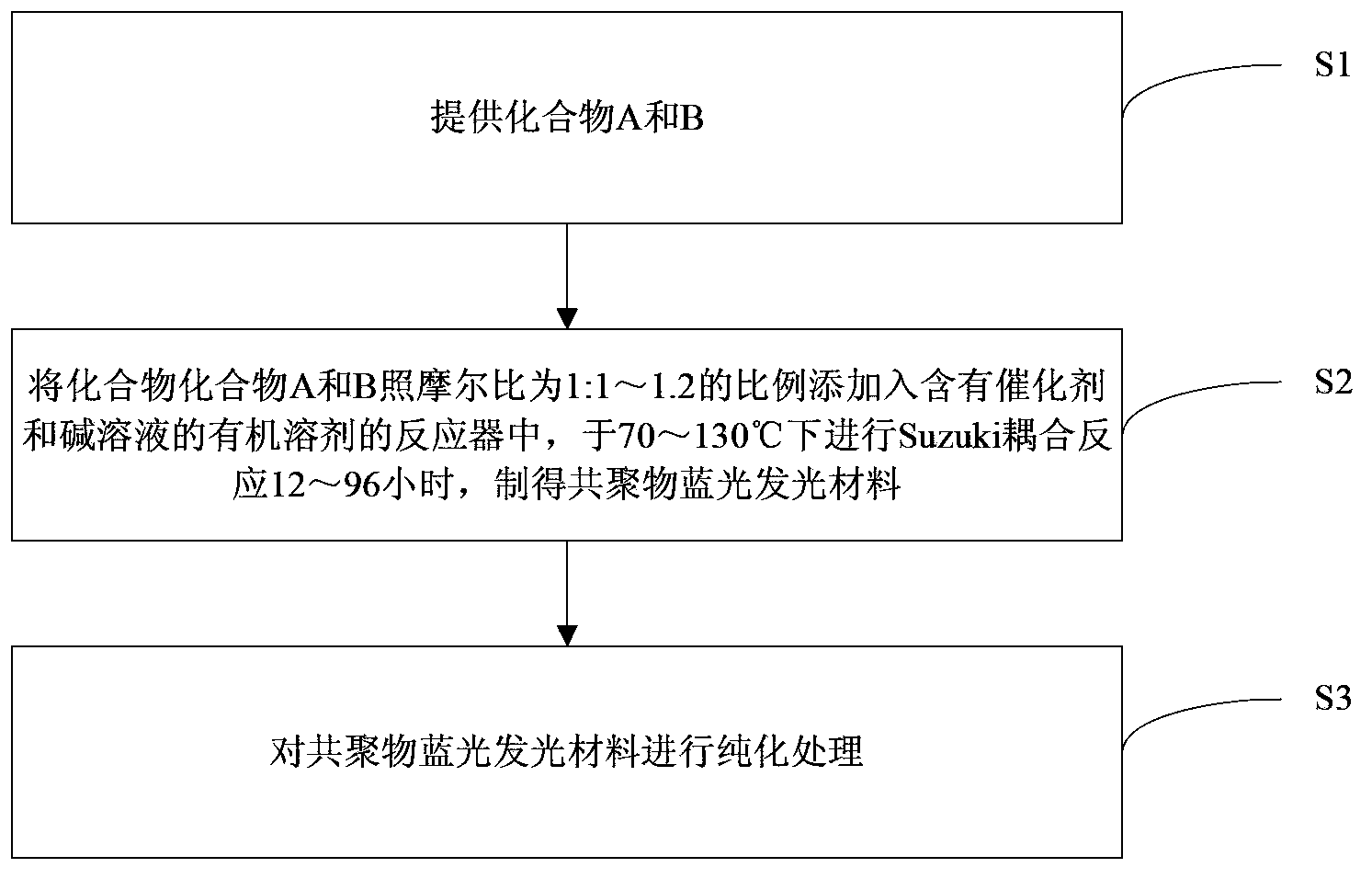
[0038] In the preparation method of the above-mentioned copolymer blue light emitting material, in step S2:
[0039] The catalyst is bistriphenylphosphine palladium dichloride or tetrakistriphenylphosphine palladium, and the molar ratio of the catalyst to compound A is 1:20 to 1:100; or
[0040] The catalyst is a mixture of organic palladium and organic phosphine ligand, the molar ratio of organic palladium to organic phosphine ligand is 1:4-8, and the molar ratio of organic palladium to compound A is 1:20-1:100 The organic palladium is palladium acetate or three dihydrobenzylacetone dipalladium; the organic phosphine ligand is tri-o-tolylphosphine or 2-bicyclohexylphosphine-2',6'-dimethoxybiphenyl;
[0041] The alkali solution is at least one of sodium carbonate solution, potassium carbonate solution and sodium bicarbonate solution; in the alkali solution, the molar ratio of alkali to compound A is 15:1-40:1;
[0042] The organic solvent is at least one selected from toluene, N,N-dim...
Embodiment 1
[0048] The electron-transporting copolymer blue-ray host material of this embodiment is poly{9,9-di-n-octyl-2,7-diylfluorene-co-2,7-diyl-4,9-diphenylanthracene Azole}(P1) (where R is n-octyl group, n=59), its structural formula is as follows:
[0049]
[0050] The preparation steps of the above polymer are as follows:
[0051] The reaction formula is as follows:
[0052]
[0053] Under the protection of argon, 9,9-di-n-octyl-2,7-dipinacol ester fluorene (128mg, 0.2mmol), 2,7-dibromo-4,9-diphenylanthrazole ( 98mg, 0.2mmol) was added to a flask containing 10ml of toluene solvent, after fully dissolved, potassium carbonate solution (2mL, 2mol / L) was added to the flask, vacuumed to remove oxygen and filled with argon, and then added bistriphenylene Phosphine dichloride palladium (5.6mg, 0.008mmol); the flask was heated to 100°C for Suzuki coupling reaction for 48h. Subsequently, the coupling reaction was stopped after cooling, and 50 ml of methanol was added dropwise to the flask for ...
Embodiment 2
[0056] The electron-transporting copolymer blue-ray host material of this embodiment is poly{9,9-dimethyl-2,7-diylfluorene-co-2,7-diyl-4,9-diphenylanthrazole }(P2) (where R is methyl, n=42), its structural formula is as follows:
[0057]
[0058] The preparation steps of the above polymer are as follows:
[0059] The reaction formula is as follows:
[0060]
[0061] Under the protection of a mixture of nitrogen and argon, the 9,9-dimethyl-2,7-dipinacol ester fluorene (134mg, 0.3mmol), 2,7-dibromo-4,9-diphenylanthracene Add azole (147mg, 0.3mmol) and 15mL of tetrahydrofuran into a 50mL two-necked flask. After fully dissolving, add a mixture of nitrogen and argon to exhaust air for about 20 minutes, and then add tetrakistriphenylphosphine palladium (4mg, 0.003mmol) Add to it, after fully dissolving, add sodium bicarbonate solution (3mL, 2mol / L). After fully ventilating the mixed gas of nitrogen and argon for about 10 minutes, the two-necked flask was added to 70 ℃ for Suzuki couplin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com