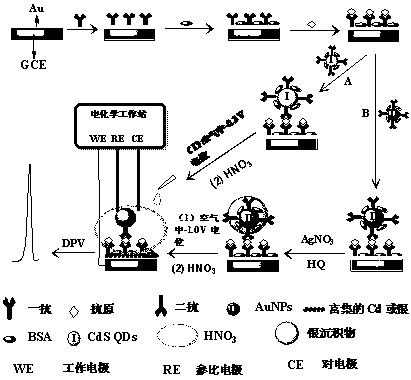
In situ anodic stripping voltammetry method based on metal labeling and bioaffinity
An anodic stripping voltammetry, metal labeling technology, applied in the direction of analyzing materials, material analysis by electromagnetic means, measuring devices, etc., can solve the problems of limiting the detection sensitivity of metal immunoelectric analysis and increasing the complexity of experimental operation, and achieves improvement. The effect of electrochemical signal output, enrichment efficiency improvement, and detection sensitivity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Gold nanoparticles (AuNPs)-labeled secondary antibodies, CdS quantum dots (CdSQDs)-labeled secondary antibodies (Ab 2 -CdS), immune electrodes were prepared according to the following method:
[0037] (1) Preparation of AuNPs-labeled secondary antibody (Ab 2 -AuNPs): in 100mL of boiling water, under vigorous stirring, add 250mL of 4% (mass concentration) HAuCl 4 , then add 2.5mL of 1% (mass concentration) sodium citrate dropwise, when the solution turns wine red, heat for another 15min. After heating was stopped, the mixture was cooled to room temperature with stirring. Add 30 mg of secondary antibody (Ab 2 ), adsorbed overnight. After centrifugation at 4800rpm at low speed for 30min, wash twice with phosphate buffered saline (PBS). Finally, the conjugate was redispersed in 1mL0.1mol / LPBS containing 1% bovine serum albumin (BSA) to increase the stability of the immunogold colloid and reduce the non-specific adsorption in the analysis. Ab prepared when not in use ...
Embodiment 2
[0061] Example 2: Electroanalysis of nucleic acid aptamers based on the method of the present invention
[0062] This example is based on the electroanalysis of nucleic acid aptamer affinity, metal labeling and anodic stripping voltammetry to detect thrombin, using the technical solution of the present invention to obtain the response in the nucleic acid aptamer electrode.
[0063] This embodiment includes the following steps:
[0064] Sample determination:
[0065] 1. Nucleic acid aptamer analysis of gold standard and silver staining:
[0066] The method of the present invention: the implementation steps of the "method of the present invention" in the "gold standard silver staining immunoassay" in Example 1 are the same.
[0067] 2. Analysis of nucleic acid aptamers labeled with CdSQDs:
[0068] The method of the present invention: the implementation steps of the "method of the present invention" in the "immunoassay of CdSQDs labeling" in Example 1 are the same.
[0069] ...
Embodiment 3
[0070] Embodiment 3: The method of the present invention based on the gold-labeled silver-stained immunoelectrode set for simultaneous detection of carcinoembryonic antigen (hCEA) and alpha-fetoprotein (hAFP) two target analytes
[0071] Sample determination: the implementation steps of the "method of the present invention" in the "gold standard silver staining immunoassay" in Example 1 are the same. Add release agent 5mL1mol / LHNO to test conditions 3 .
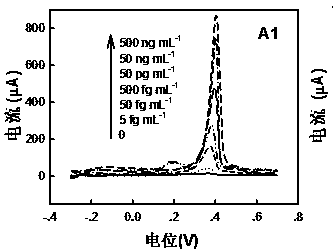
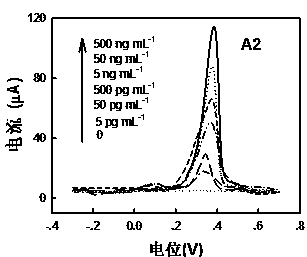
[0072] Analysis of measurement results: discuss in conjunction with Figure 5. Fig. 5 shows the signal response standard curve of the gold-labeled immune electrode for detecting different concentrations of antigens based on the anodic stripping voltammetry of the electrode group according to the present invention. It can be seen from the figure that as the antigen concentration increases, the current response also increases. The linear range of hCEA and hAFP is 4fg / mL~400ng / mL, and the lower detection limits are 2.8fg / mL and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com