Method for preparing lithium oxalyldifluoroborate
A technology of lithium difluorooxalate borate and difluorooxalate borate, which is applied in the field of lithium battery electrolyte material manufacturing, can solve the problems of long reaction time, low yield and high preparation cost, and achieve short reaction time, high yield and high preparation The effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
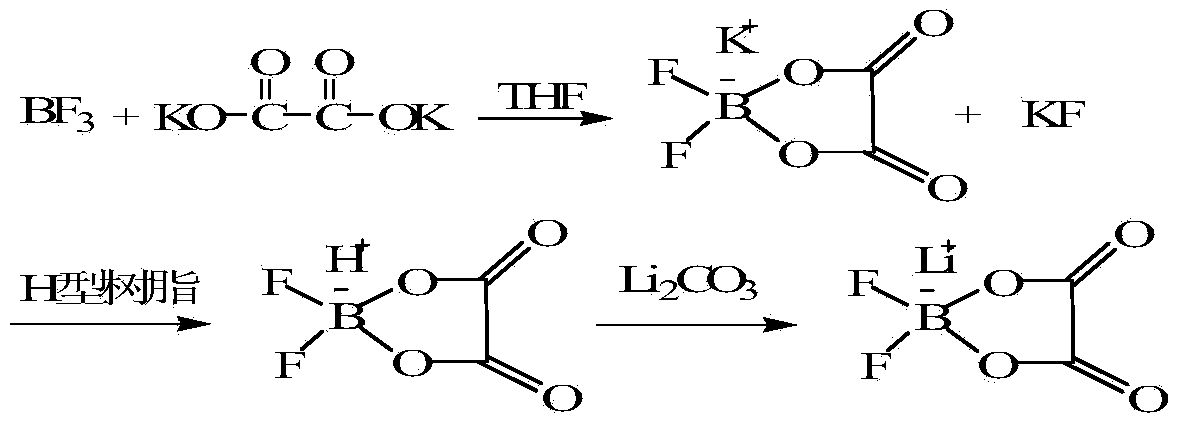
[0019] Embodiment 1 uses boron trifluoride and potassium oxalate as raw materials to prepare lithium difluorooxalate borate
[0020]
[0021] Step 1: Add 166g (1mol) of anhydrous potassium oxalate and 200mL of dry tetrahydrofuran (THF) into a 1L autoclave, feed 68g (1mol) of boron trifluoride gas, heat the reaction system to 60°C, and control the reaction pressure After reacting at 0.2MPa for 6 hours, let it stand still, remove the insoluble matter in the reaction system by suction filtration under reduced pressure, and obtain a tetrahydrofuran solution of potassium difluorooxalate borate.
[0022] Step 2: Potassium difluorooxalate borate is obtained as a white solid after removing the solvent under reduced pressure. Potassium difluorooxalate borate is dissolved in water, flowed through the acidified cation exchange resin, collected to obtain difluorooxalate borate solution, add 37g (0.5 mol) Lithium Retard, stirred and reacted for 1 hour at room temperature and under the c...
Embodiment 2
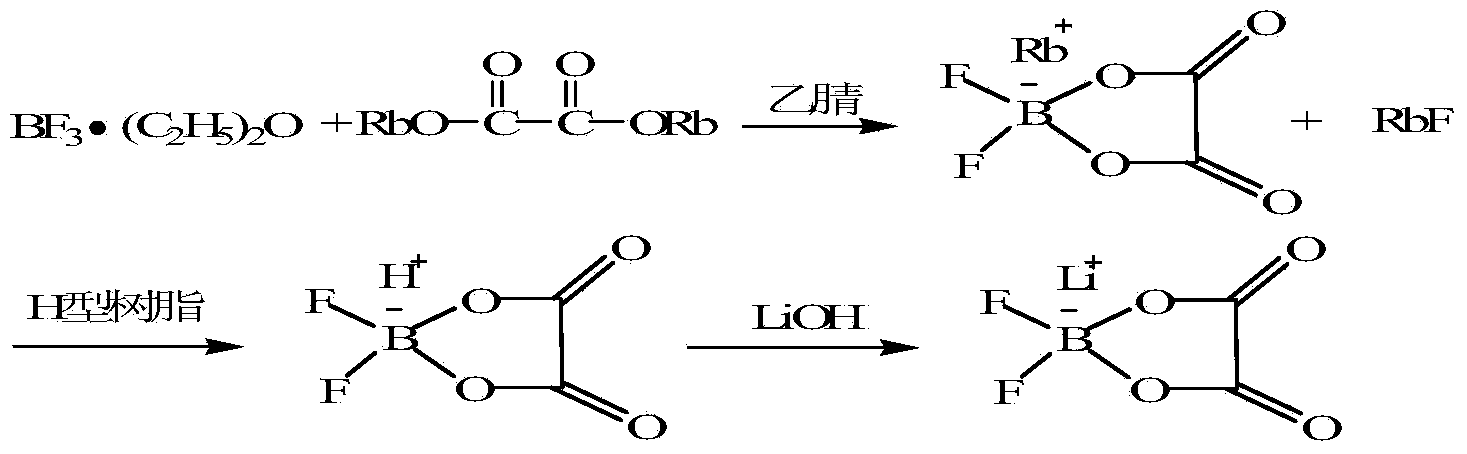
[0025] Example 2 Using boron trifluoride ether solution and rubidium oxalate as raw materials to prepare lithium difluorooxalate borate
[0026]
[0027]Step 1: Add 258g (1mol) anhydrous rubidium oxalate, 150mL dry acetonitrile and 170g (1.2mol) boron trifluoride ether solution into a 1L autoclave, heat the reaction system to 50°C, and control the reaction pressure at 0.2MPa , After reacting for 8 hours, let it stand still, remove the insoluble matter in the reaction system by suction filtration under reduced pressure, and obtain an acetonitrile solution of rubidium difluorooxalate borate.
[0028] Step 2: After the solvent is removed under reduced pressure, rubidium difluorooxalate borate is obtained as a white solid, and rubidium difluorooxalate borate is dissolved in water, and the cation exchange resin after the acidification treatment is flowed through, and the difluorooxalate borate solution is collected, and 24 g (1mol ) lithium hydroxide, stirred and reacted for 2 h...
Embodiment 3
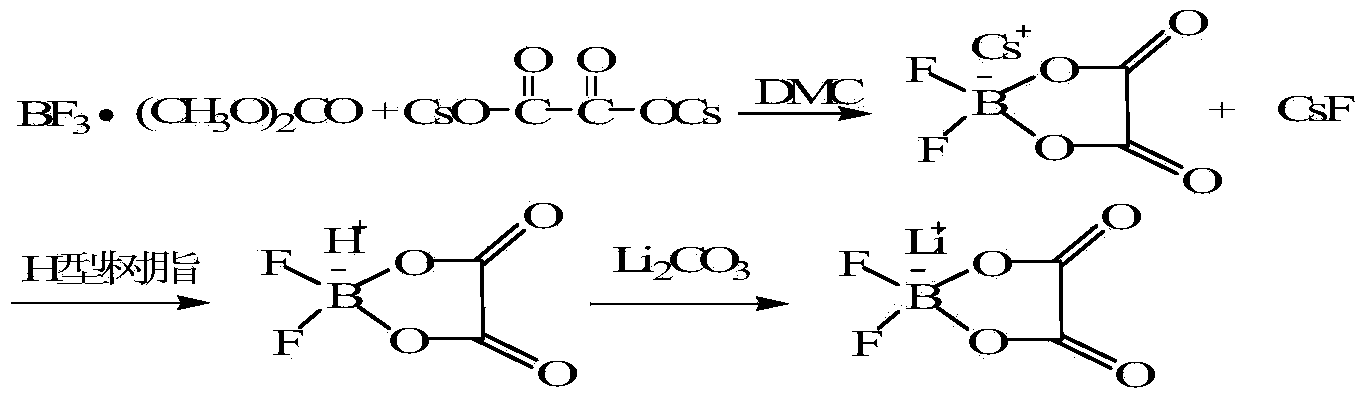
[0031] Example 3 Using boron trifluoride dimethyl carbonate solution and cesium oxalate as raw materials to prepare lithium difluorooxalate borate
[0032]
[0033] Step 1: Add 354g (1mol) cesium oxalate anhydrous in the autoclave of 2L, 200mL dry dimethyl carbonate (DMC) and 237g (1.5mol) boron trifluoride dimethyl carbonate solution, the reaction system is heated up to 70°C, the reaction pressure is controlled at 0.2MPa, after 6 hours of reaction, let it stand still, remove the insoluble matter in the reaction system by suction filtration under reduced pressure, and obtain a dimethyl carbonate solution of cesium difluorooxalate borate.
[0034] Step 2: After the solvent is removed under reduced pressure, cesium difluorooxalate borate is obtained as a white solid, and cesium difluorooxalate borate is dissolved in an appropriate amount of water, and the acidified cation exchange resin is flowed through to collect the difluorooxalate borate solution, and 59 g ( 0.8mol) of li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com