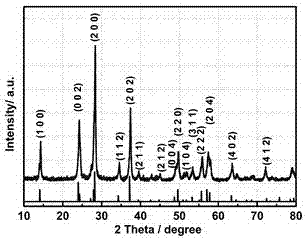
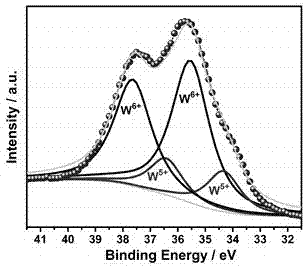
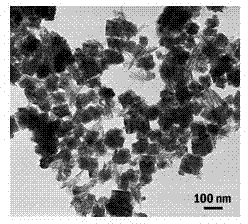
Preparation method of as-reduced ammonium tungsten bronze nanoparticles
A nanoparticle and tungsten bronze technology, which is applied in the direction of nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of excessive sample particle size, easy decomposition, and decreased crystallization performance, and achieve uniform particle shape and synthesis steps. Simple, narrow particle size distribution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 36ml oleic acid and 0.4g WCl to 100ml hydrothermal reaction kettle 6 After powdering, stir and mix at room temperature; after it is completely dissolved, add 4 ml of oleylamine, then seal the reaction vessel, and place it in an oven at 200 °C for 24 h to crystallize. After cooling to room temperature, it was separated by centrifugation, washed alternately with 30 mL deionized water and 30 mL absolute ethanol three times, and dried in vacuum to obtain ammonium tungsten bronze blue powder, which was a square-shaped ammonium tungsten bronze particle with an average diameter of 80nm.
Embodiment 2
[0028] Add 25ml oleic acid and 0.4g WCl to 100ml hydrothermal reaction kettle 6 After powdering, stir and mix at room temperature; after it is completely dissolved, add 15 ml of oleylamine, then seal the reaction vessel, and place it in an oven at 200 °C for 24 h to crystallize. After cooling to room temperature, centrifuge, wash with 30 mL deionized water and 30 mL absolute ethanol alternately three times, and vacuum dry to obtain ammonium tungsten bronze blue powder, which is rod-shaped ammonium tungsten bronze particles with an average diameter of 50nm. The nanorods have an average length of 350 nm.
Embodiment 3
[0030] Dissolve 0.4g of tungsten chloride in 32ml of oleic acid, stir well until it is completely dissolved, then add 8ml of oleylamine, mix until uniform, move to a supercritical reaction kettle, and crystallize at 350°C for 1 hour. After the reaction, the powder The sample was centrifuged, washed, and vacuum-dried at 60°C for 6 hours to obtain ammonium tungsten bronze blue powder, which was square ammonium tungsten bronze particles with an average diameter of 110nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| The average diameter | aaaaa | aaaaa |
| Average length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com