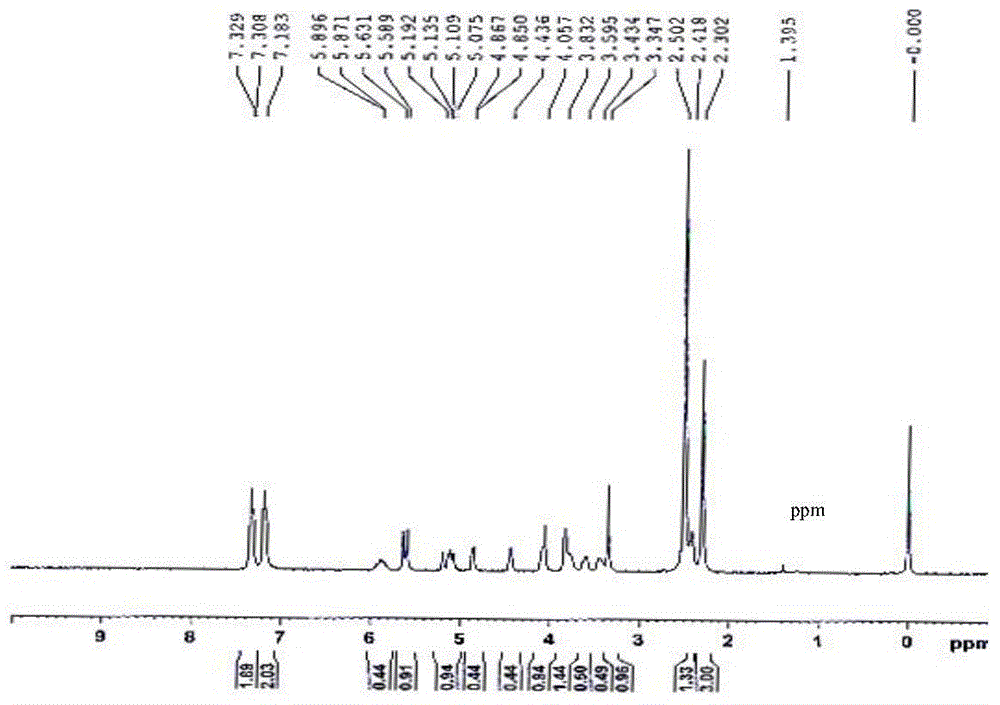
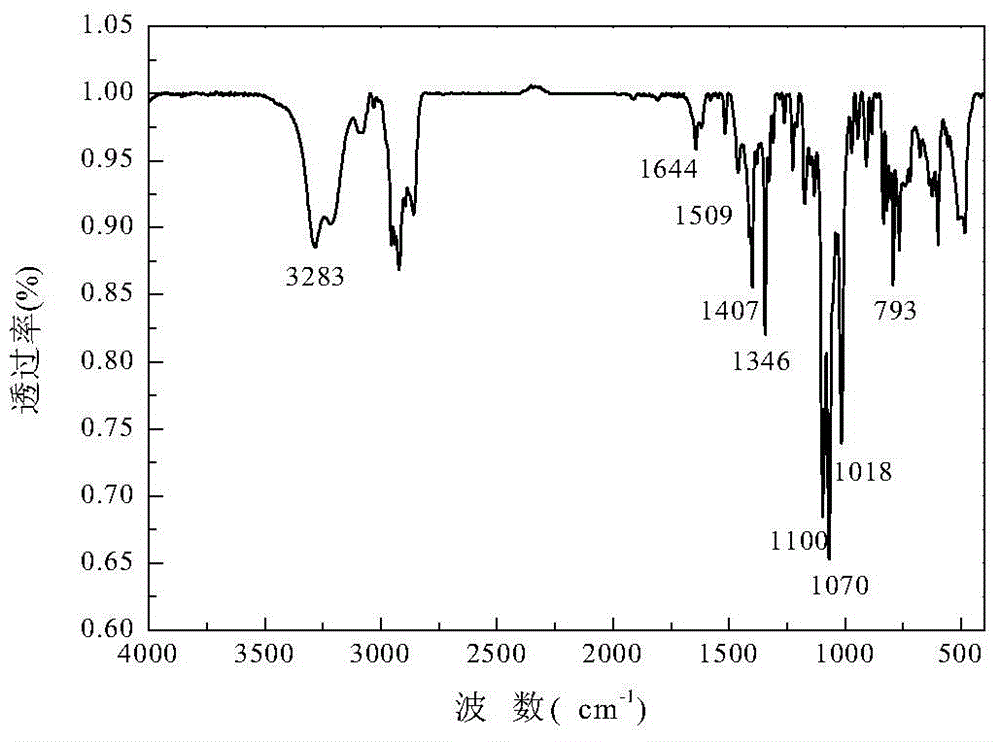
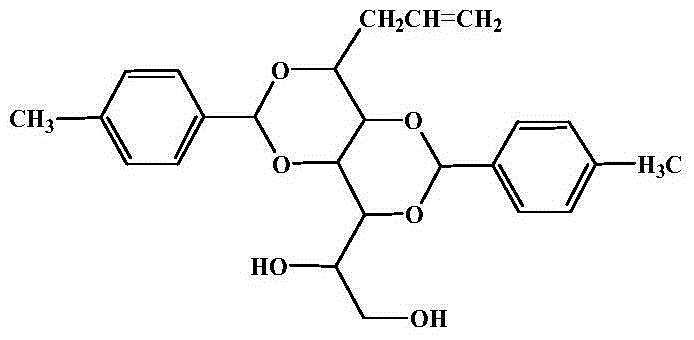
Preparation method of 1,3-2,4-di(p-methyl)benzylidene-1-allyl sorbitol
An allyl sorbitol and p-methyl technology, which is applied in the field of preparation of acetal compounds, can solve the problems of easy decomposition, instability, and many bubbles, and achieves easy control of the reaction process, mild reaction conditions, and reduced production. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of 1-allyl sorbitol
[0025] The configuration concentration is 0.5mol / L HBr alcohol aqueous solution, wherein the volume ratio of ethanol and water is 1:9. Glucose 15.00g, allyl bromide 10.10g, Sn powder 4.95g, SnCl 2 9.40g, 420ml of the above-mentioned 0.5mol / L HBr alcohol aqueous solution was placed in a 500ml three-necked flask, stirred at room temperature for 48h; after the reaction was completed, it was neutralized with a saturated NaOH ethanol solution to pH=7, and a large amount of white precipitates were formed during the dropwise addition; The suspension was filtered to remove solids; the filtrate was removed by rotary evaporation to obtain the milky white intermediate product 1-allyl sorbitol.
[0026] Preparation of 1,3-2,4-di(p-methyl)benzylidene-1-allyl sorbitol
[0027] Put 18.5g of the intermediate product, 20.0g of p-toluene aldehyde, 1.2g of p-toluenesulfonic acid, 50ml of solvent cyclohexane, and 90ml of accelerator methanol in a 500ml t...
Embodiment 2
[0031] Preparation of 1-allyl sorbitol
[0032] The configuration concentration is 0.5mol / L HBr alcohol aqueous solution, wherein the volume ratio of ethanol and water is 1:4. Glucose 15.00g, allyl bromide 10.10g, Sn powder 4.95g, SnCl 2 9.40g, 420ml of the above-mentioned 0.5mol / L HBr alcohol aqueous solution was placed in a 500ml three-necked flask, stirred at room temperature for 48h; after the reaction was completed, it was neutralized with a saturated NaOH ethanol solution to pH=7, and a large amount of white precipitates were formed during the dropwise addition; The suspension was filtered to remove solids; the filtrate was removed by rotary evaporation to obtain the milky white intermediate product 1-allyl sorbitol.
[0033] Preparation of 1,3-2,4-di(p-methyl)benzylidene-1-allyl sorbitol
[0034]Put 18.5g of intermediate product, 20.0g of p-toluene aldehyde, 1.2g of p-toluenesulfonic acid, 50ml of solvent cyclohexane, and 90ml of accelerator methanol in a 500ml three-...
Embodiment 3
[0036] Preparation of 1-allyl sorbitol
[0037] The configuration concentration is 0.5mol / L HBr alcohol aqueous solution, wherein the volume ratio of ethanol and water is 1:1. Glucose 15.00g, allyl bromide 10.10g, Sn powder 4.95g, SnCl 2 9.40g, 420ml of the above-mentioned 0.5mol / L HBr alcohol aqueous solution was placed in a 500ml three-necked flask, and stirred at room temperature for 48h; after the reaction was completed, neutralized with saturated NaOH ethanol solution to pH=7, and a large amount of white precipitate was formed during the dropwise addition; The suspension was filtered to remove solids; the filtrate was removed by rotary evaporation to obtain the milky white intermediate product 1-allyl sorbitol.
[0038] Preparation of 1,3-2,4-di(p-methyl)benzylidene-1-allyl sorbitol
[0039] Put 18.5g of intermediate product, 20.0g of p-toluene aldehyde, 1.2g of p-toluenesulfonic acid, 50ml of solvent cyclohexane, and 90ml of accelerator methanol in a 500ml three-necked...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com