Oxygen-carrying liposome with low surface tension and preparation method thereof
A liposome and oxygen-carrying technology, applied in the direction of liposome delivery, halogenated hydrocarbon active ingredients, amine active ingredients, etc., can solve high surface tension, difficult alveolar surface spreading, and inability to provide oxygen-carrying lipids problems such as bulk, to achieve the effect of high oxygen content and low surface tension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
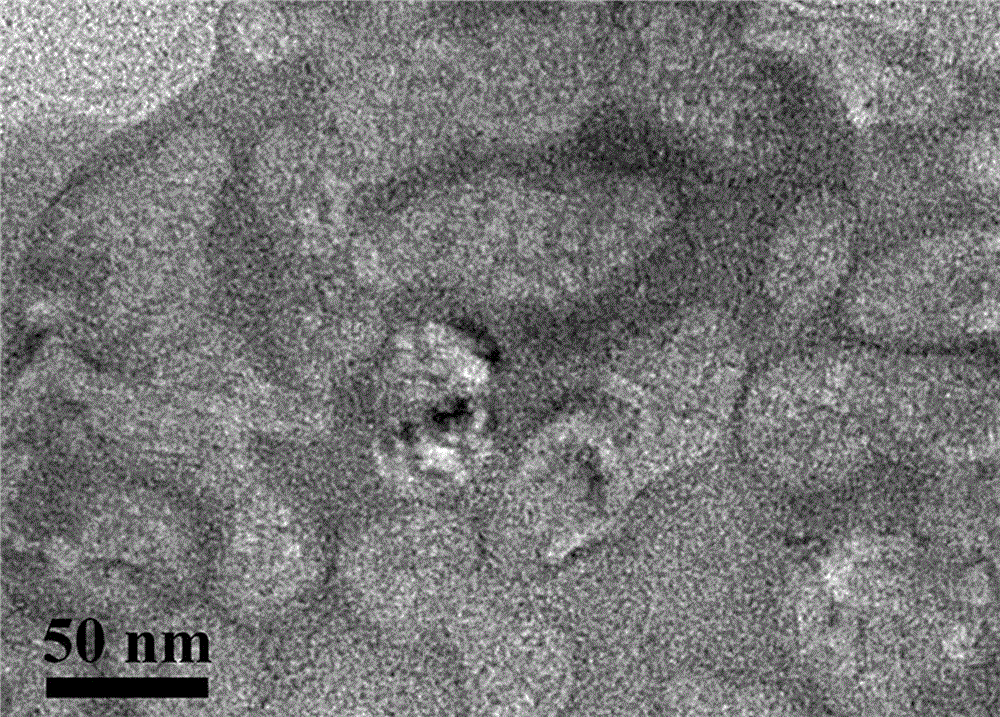
[0025] Example 1: Preparation of oxygen-carrying liposomes.
[0026] In parts by weight, the formula is as follows:
[0027] 2000 parts of normal saline;
[0028] 200 parts of soybean lecithin;
[0029] Perfluorooctyl bromide 200 parts;
[0030] 1000 parts of dichloromethane;
[0031] 5 parts of palmitic acid;
[0032] 2 parts of tyloxapol.
[0033] Preparation Process:
[0034] (1) In parts by weight, 200 parts of soybean lecithin, 5 parts of palmitic acid, and 2 parts of tyloxapol were dissolved in 2000 parts of ether, and ultrasonically mixed evenly;
[0035] (2) Add 200 parts of perfluorooctyl bromide to the ether solution, and continue to mix evenly by ultrasonic;
[0036] (3) Slowly add the solution obtained in step (2) dropwise to 2000 parts of normal saline, stir the solution (stirring speed is 1000 rpm), and stir until the ether is completely volatilized for about 2 hours. That is, oxygen-carrying liposomes encapsulating perfluorooctyl bromide can be obtained....
Embodiment 2
[0037] Example 2: Preparation of oxygen-carrying liposomes.
[0038] In parts by weight, the formula is as follows:
[0039] 2000 parts of phosphate buffer;
[0040] 200 parts of egg yolk lecithin;
[0041] FC-77 (a mixture of perfluorooctane and perfluorocyclooctyl ether) 200 parts;
[0042] 1000 parts of ether;
[0043] 1 part cetyl alcohol;
[0044] 1 part of tyloxapol.
[0045] Preparation Process:
[0046] (1) In parts by weight, 200 parts of soybean lecithin, 2 parts of cetyl alcohol, and 1 part of tyloxapol were dissolved in 1000 parts of ether, and ultrasonically mixed evenly;
[0047] (2) Add 200 parts of FC-77 (a mixture of perfluorooctane and perfluorocyclohexyl ether) to the ether solution, and continue to mix evenly by ultrasonic;
[0048] (3) Slowly add the solution obtained in step (2) dropwise to 2000 parts of normal saline, stir the solution (stirring speed is 1000 rpm), and stir until the ether is completely volatilized for about 2 hours. That is, oxy...
Embodiment 3
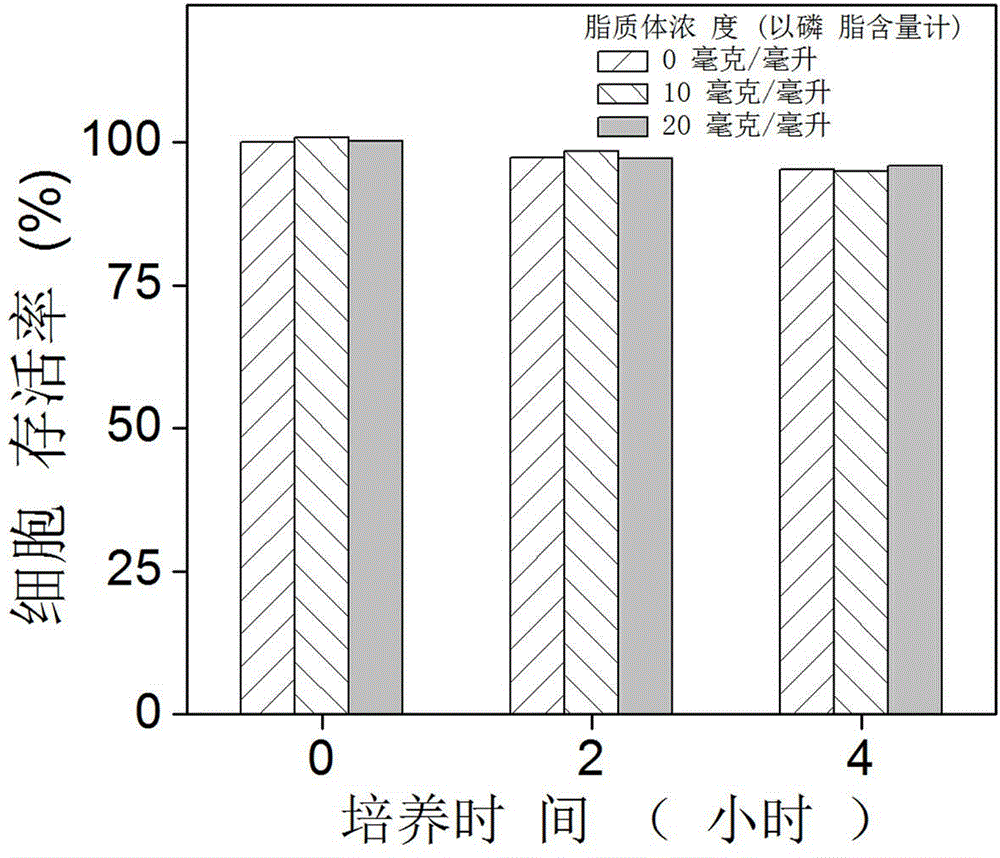
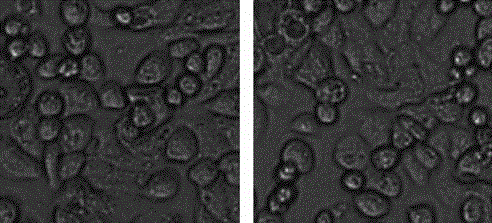
[0049] Example 3 Toxicity evaluation of oxygen-carrying liposomes.
[0050] Take monolayer cultured HEK293 epithelial cells in the logarithmic growth phase, digest the monolayer cultured cells with 0.25% trypsin and 0.025% ethylenediaminetetraacetic acid disodium salt solution, and prepare with DMEM cell culture medium containing 10% fetal bovine serum Single cell suspension, 1×10 per well 4 Cells were seeded in a 96-well plate with a volume of 100 microliters per well, and the culture plate was moved into a carbon dioxide incubator, and cultured overnight at 37°C, 5% carbon dioxide and saturated humidity to allow the cells to adhere to the wall. On the next day, a series of fluorocarbon oxygen-carrying liposome solutions (using the oxygen-carrying liposome prepared in Example 2) with different concentrations were prepared with phosphate-containing solution. Aspirate the culture medium in the culture dish, add the oxygen-carrying liposome solution, incubate at 37°C for differen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com