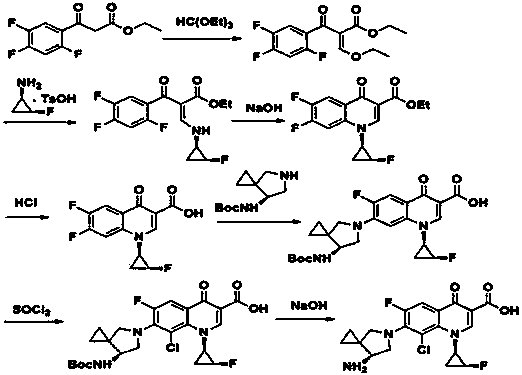
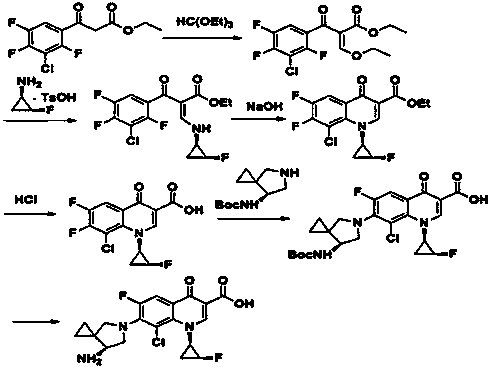
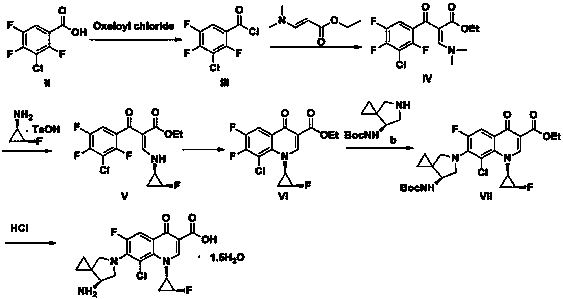
Sitafloxacin preparation method
A technology of sitafloxacin and compounds, applied in the field of new chemical drug preparation technology, can solve the problems of large environmental pollution, cumbersome post-processing, high cost, etc., and achieve the effect of reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Synthesis of N, N-dimethylamino ethyl acrylate
[0038] The reaction equation is as follows:
[0039]
[0040] References: Wang Weiqiang, Gu Haining, etc. Synthesis of N,N-Dimethylaminoethyl Acrylate. Chemical Production and Technology, 2008, 15(4): 29-33.
[0041] Examples are as follows
[0042] In a dry reaction vessel, add 400 mL of toluene and 58 g of sodium ethoxide, cool to -5~0°C, add dropwise a mixture of 82 g of ethyl acetate and 99 g of ethyl formate, which have been re-evaporated, under 0°C. After reacting for 10-11h, continue to add 60g of dimethylamino hydrochloride, stir and react at 25-30℃ for 10-11h. After the reaction is completed, it is filtered, and the filtrate is rotary evaporated to recover toluene. The residue is distilled to obtain the target product, N,N-di The ethyl methaminoacrylate is about 75g, and the total yield is about 58%.
[0043]
Embodiment 2
[0044] Example 2 Synthesis of (s)-(-)-7-tert-butoxycarbonylamino-5-azaspiro[2.4]heptane
[0045] The reaction equation is as follows:
[0046]
[0047] Examples are as follows
[0048] Add 350g of compound of formula a, 3.5L of methanol, 80g of 10%Pd / C to the reaction vessel, add 180mL of formic acid dropwise, stir for 7h at room temperature, after the reaction is complete, filter with suction, concentrate the filtrate to dryness, add dichloromethane and water to the remainder , Adjust pH 11-12 with 10% sodium hydroxide at 0-5°C, separate the layers, extract the aqueous layer twice with dichloromethane, combine the organic layers, wash with saturated sodium chloride solution, and dry the organic layer with anhydrous sodium sulfate. The desiccant was filtered off, and the filtrate was concentrated to dryness under reduced pressure to obtain a yellow oil. When cooled, about 220 g of colorless crystals were obtained with a yield of 90%.
[0049]
Embodiment 3
[0050] Example 3 Preparation of crude sitafloxacin
[0051] Dissolve 2,4,5-trifluoro-3-chlorobenzoic acid (84g, 0.4mol) in DMF 252mL, add oxalyl chloride (55.8g, 0.44mol), and react at 35°C for 2h. Dissolve triethylamine (48.5g, 0.48mol) and N,N-dimethylaminoethyl acrylate (58g, 0.4mol) with about 126mL of dichloromethane and add dropwise to the above reaction solution, warm to 50°C, and stir. The reaction was carried out for 2.5 hours, TLC monitored the reaction to be complete, cooled to room temperature, and acidified by adding acetic acid (27g, 0.44mol).
[0052] Mix (1R, 2S)-(-)-cis-1-amino-2-fluorocyclopropane p-toluenesulfonate (99g, 0.4mol) and 200mL of dichloromethane, reduce the temperature to -10°C, stir, and slowly Add triethylamine (47g, 0.46mol) dropwise, control the temperature below 0°C, after the dropwise addition is complete, keep stirring for 18min, add dropwise to the above reaction solution, continue the reaction at 30°C for 1h, after the reaction is completed,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com