Pharmaceutical composition for treating chronic gastroenteritis and preparation method thereof
A technology for chronic gastroenteritis and a composition is applied in the field of pharmaceutical compositions for treating chronic gastroenteritis and the field of preparation thereof, which can solve the problems of complex disease, aggravate gastric symptoms of chronic gastritis, and be difficult to cure, and achieve improved quality of life and adverse reactions. and the effect of reducing side effects and solving the root cause of the disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
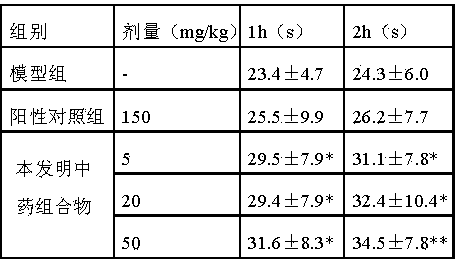
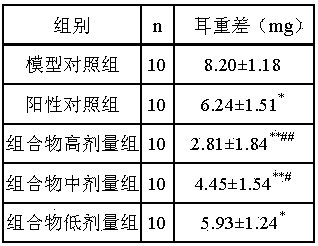
Image
Examples
Embodiment 1
[0028] Example 1 Traditional Chinese medicine composition and preparation process of the present invention
[0029] The composition of the prescription is as follows:
[0030] 8 parts of Veratrum, 5 parts of Chuanxiong, 5 parts of Paeoniae Alba, 3 parts of Fructus Aurantii, 10 parts of Atractylodes Rhizome, 5 parts of Angelica, 10 parts of Codonopsis, 3 parts of Gynostemma, 10 parts of Acanthopanax, 5 parts of Danshen, 8 parts of Poria cocos, Rehmannia glutinosa 5 parts, 10 parts of kudzu root, 3 parts of asarum, 5 parts of mint, 3 parts of cocklebur, 3 parts of Ligustrum lucidum, 5 parts of epimedium, 10 parts of Sophora japonica, 10 parts of dogwood, 3 parts of woody fragrance, 5 parts of dodder seed , 5 parts of purslane, 5 parts of astragalus, 10 parts of licorice.
[0031] Preparation method: Take the prescription amount of the above-mentioned Chinese medicinal materials and crush them into coarse powder, add 40% ethanol solution with a volume concentration of 4 times ...
Embodiment 2
[0032] Embodiment 2 Chinese medicine composition of the present invention and preparation technology
[0033] The composition of the prescription is as follows:
[0034]20 parts of Veratrum, 15 parts of Chuanxiong, 15 parts of Paeoniae Alba, 10 parts of Citrus aurantium, 25 parts of Atractylodes macrocephala, 15 parts of Angelica sinensis, 20 parts of Codonopsis pilosula, 10 parts of Jiaogulan, 20 parts of Acanthopanax, 15 parts of Salvia miltiorrhiza, 20 parts of Poria cocos, Rehmannia glutinosa 12 parts, Pueraria 20 parts, Asarum 10 parts, Mint 15 parts, Cocklebur 10 parts, Ligustrum lucidum 10 parts, Epimedium 12 parts, Sophora japonica 15 parts, Dogwood 20 parts, Woody 10 parts, Cuscuta 15 parts , 15 parts of purslane, 25 parts of astragalus, 20 parts of licorice.
[0035] Preparation method: Take the prescription amount of the above-mentioned Chinese medicinal materials and crush them into coarse powder, add 90% ethanol solution according to the volume concentration of...
Embodiment 3
[0036] Embodiment 3 Chinese medicine composition of the present invention and preparation technology
[0037] The composition of the prescription is as follows:
[0038] 15 parts of Veratrum, 10 parts of Rhizoma Chuanxiong, 10 parts of Paeoniae Alba, 7 parts of Fructus Aurantii, 18 parts of Atractylodes Rhizome, 10 parts of Angelica sinensis, 15 parts of Codonopsis, 7 parts of Jiaogulan, 15 parts of Acanthopanax, 10 parts of Danshen, 14 parts of Poria cocos, Rehmannia glutinosa 9 parts, 15 parts of kudzu root, 7 parts of asarum, 10 parts of mint, 7 parts of cocklebur, 6 parts of Ligustrum lucidum, 8 parts of epimedium, 13 parts of Sophora japonica, 15 parts of dogwood, 7 parts of woody fragrance, 10 parts of dodder seed , 10 parts of purslane, 20 parts of astragalus, 15 parts of licorice.
[0039] Preparation method: Take the prescription amount of the above-mentioned Chinese medicinal materials and crush them into coarse powder, add 70% ethanol solution according to the vo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com