Preparation method for quantum dot labelled immunochromatographic test strip
An immunochromatographic test strip and quantum dot technology, applied in the field of medical immunodetection, can solve the problems of low sensitivity and low accuracy, and achieve the effects of good luminescence stability, narrow emission peak and symmetrical peak shape.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: a kind of quantum dot labeled immunochromatographic test paper, is provided with plastic plate, nitrocellulose membrane, glass cellulose membrane A, quantum dot labeled glass cellulose membrane B of Japanese encephalitis virus IgG monoclonal antibody, absorbent paper, Described glass cellulose film A is the glass cellulose film of buying on the market without spot;
[0033] Wherein, the plastic plate is pasted with glass cellulose membrane A, quantum dot-labeled glass cellulose membrane B of Japanese encephalitis virus IgG monoclonal antibody, nitrocellulose membrane, and absorbent paper in sequence;
[0034] Wherein, there are Japanese encephalitis virus polyclonal antibody and rabbit anti-mouse secondary antibody at one end of the nitrocellulose membrane, so as to form detection zone T and quality control zone C;
[0035] Wherein, the JEV IgG monoclonal antibody labeled with quantum dots is located at one end of the glass cellulose membrane B, correspond...
Embodiment 2
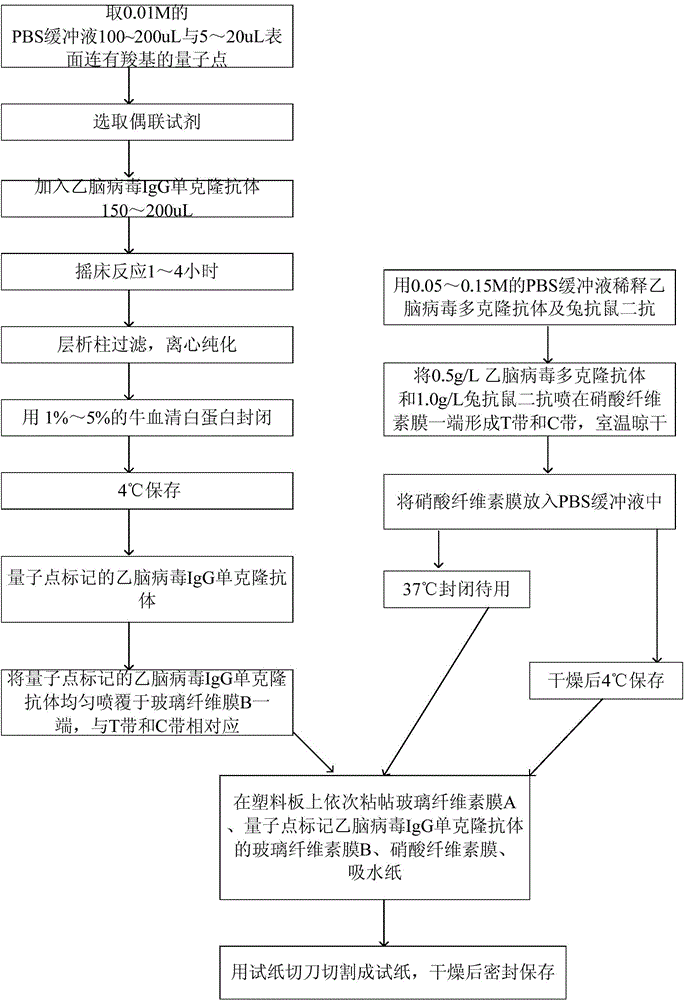
[0041] Embodiment 2: the preparation method of test paper as mentioned above, as figure 1 shown, including the following steps:
[0042] (1) Coupling of quantum dots and Japanese encephalitis virus IgG monoclonal antibody:
[0043] Take 100-200uL of 0.01M PBS buffer and 5-20uL of quantum dots with carboxyl groups on the surface;
[0044] A coupling reagent is selected, and the coupling reagent is selected from hydroxysulfosuccinic acid imide, 1-(3-dimethylaminopropyl)-3 ethylcarbodiamine hydrochloride;
[0045] Add 150-200uL of Japanese encephalitis virus IgG monoclonal antibody;
[0046] Shaker reaction for 1 to 4 hours;
[0047] Column filtration, centrifugal purification;
[0048] Block with 1% to 5% bovine serum albumin;
[0049] Store at 4°C;
[0050] (2) Preparation of test paper:
[0051] Dilute JEV polyclonal antibody and rabbit anti-mouse secondary antibody with 0.05-0.15M PBS buffer, spray 0.5g / L JEV polyclonal antibody and 1.0g / L rabbit anti-mouse secondary a...
Embodiment 3
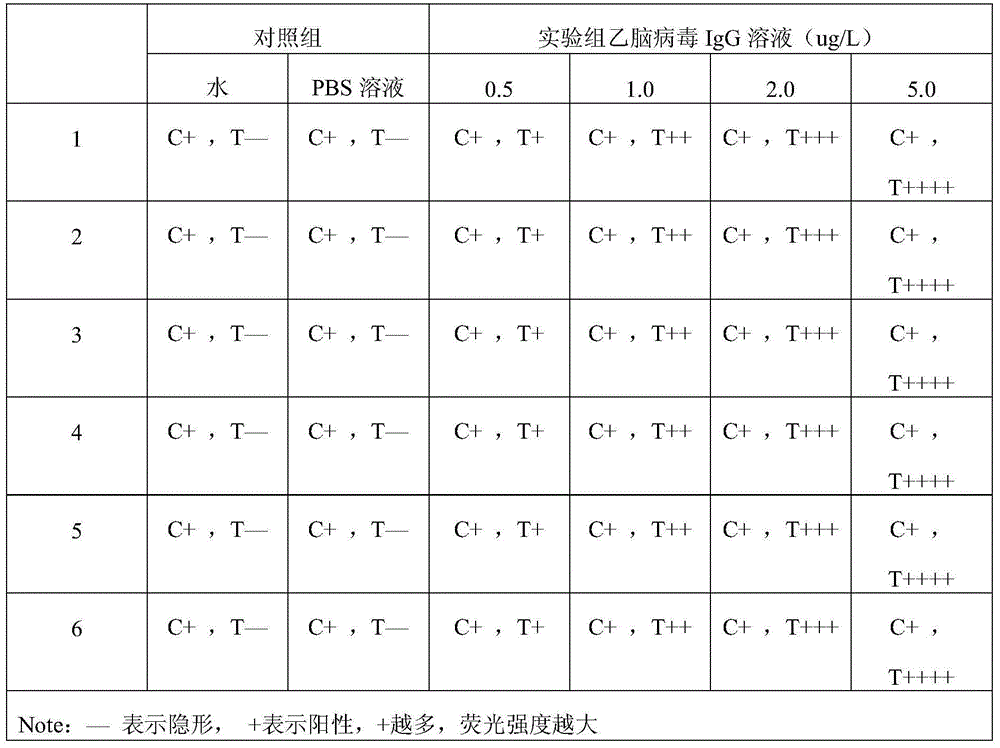
[0058] Embodiment 3: detect the IgG monoclonal antibody of Japanese encephalitis virus with described test paper, comprise the following steps: spot sample on the assembled test paper near one end of IgG monoclonal antibody of Japanese encephalitis virus, react after 5min, analyze in ultraviolet Observation results in the instrument. PBS buffer solution and normal human blood were used as blank controls.
[0059] Result judgment: under the premise that the C band shows a red fluorescent band, the intensity of the fluorescent band of the T band is visually compared with the blank. The weaker the fluorescence, the lower the concentration of the tested substance in the test solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com