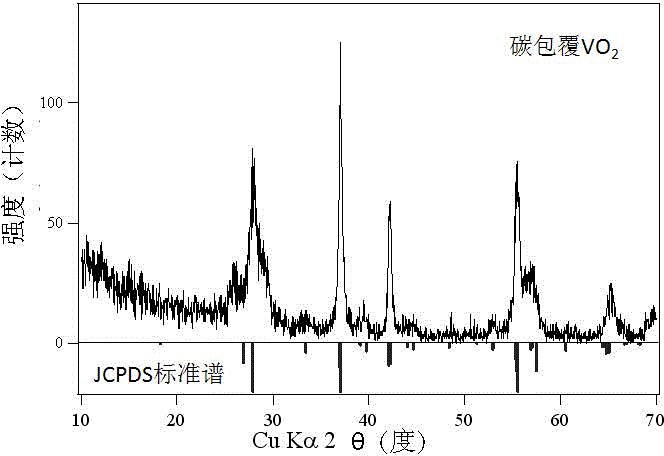
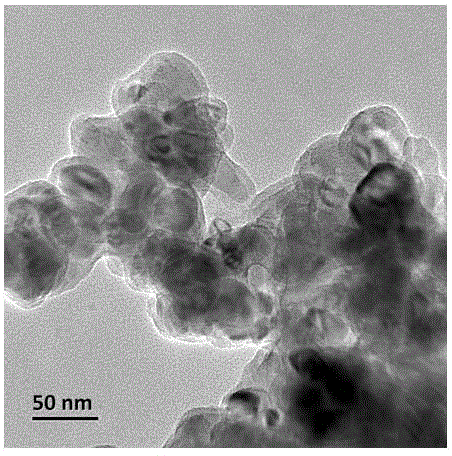
Carbon-coated vanadium dioxide nanoparticles and preparation method thereof
A vanadium dioxide nano, vanadium dioxide technology, applied in the field of carbon-coated vanadium dioxide nanoparticles and its preparation, can solve the problems of reducing material stability, difficult process control, easy to break or break, etc., to improve stability , excellent optical properties, cheap method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Validation of Feasibility of Carbon-coated Vanadium Dioxide Nanopowders Using Optical Theory
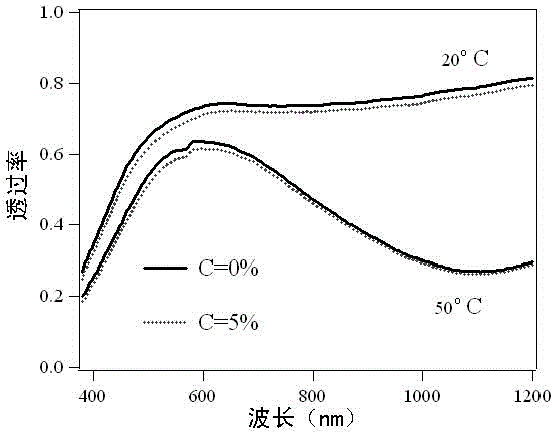
[0057]Design spherical or spherical nano vanadium dioxide powder with a size of less than 100nm, with a volume fraction of 2%, and a volume fraction of coated carbon elements of 0 and 5% of the volume fraction of vanadium dioxide, and disperse it in PET resin Among them, a PET film dispersion system with a thickness of 3 microns was formed respectively. Using the effective medium approximation theory (Effective Medium Approximation), select the Maxwell-Garnet theoretical calculation model, and use the optical constants of various related substances to calculate the equivalent optical constants of the system. and near-infrared) range of transmittance spectra, and compared with each other.
[0058] figure 1 for the calculation result. The results show that the effect on the optical properties of the vanadium dioxide nanopowder after coating with a carbon source with a carbon ...
Embodiment 2
[0060] 1) Preparation of rutile phase vanadium dioxide nanopowder: add 5% by weight hydrazine hydrate (N 2 h 4 -H 2 O, Wako Pure Chemical Co., Ltd. special reagent) 640 grams, add 120 grams of vanadium pentoxide powder (V 2 o 5 , Wako Pure Chemicals Co., Ltd.), 1.80 g tungsten oxide (WO 3 , Wako Pure Chemicals Co., Ltd.), and put it into a stainless steel hydrothermal reaction vessel with a magnetic stirring mechanism, and heated at 260 ° C for 24 hours for hydrothermal reaction (the obtained solid was verified to be pure by subsequent XRD analysis, etc. Rutile phase vanadium dioxide nanopowder);
[0061] 2) In-situ carbon coating of rutile-phase vanadium dioxide nano-powder: wait for the temperature of the reaction liquid containing rutile-phase vanadium dioxide nano-powder to drop below 100°C, then add 10 grams of glucose (Sinopharm analytically pure chemical reagents), close the reactor, keep it at 180°C for 8 hours under the condition of full stirring and cool to room...
Embodiment 3
[0068] 1) Prepare rutile phase vanadium dioxide nanopowder: prepare NH 4 VO 3 (N 2 h 4 -H 2 O, Wako Pure Chemical Co., Ltd. special grade reagent) aqueous solution with a mass percentage of 5%, add hydrazine hydrate with a molar mass ratio of 1.2, and add an appropriate amount of WO according to W:V=1.5% atomic percentage 3 , put it into a 100-liter stainless steel magnetic stirring hydrothermal reaction vessel, heat at 270°C for 24 hours for hydrothermal reaction, and after cooling, filter and wash the reaction precipitate, dry at 80°C for 24 hours to obtain rutile phase dioxide Vanadium nano-powder, and put into drying oven for subsequent use;
[0069] 2) Carry out ex-situ carbon coating on the rutile phase vanadium dioxide nanopowder: Weigh 100 grams of the above rutile phase vanadium dioxide nanopowder, together with 4000ml deionized water and 12g sucrose (Sinopharm analytically pure) Put it into a stainless steel magnetic stirring hydrothermal reaction vessel with a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com