Preparation method of double-end based amide type glycine betaine surfactant and application thereof
A double-head group amide-type, surfactant technology, applied in the field of colloid and interface chemistry, surfactants, can solve the problem that does not involve the preparation and application of double-head group amide-type betaine surfactants, and does not involve double-head group Solve the problems of amide-type betaine surfactant and other problems, achieve good solubilization effect and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: intermediate N , N -Synthesis of bis-(dimethylaminopropyl)dodecylamide
[0028] It is 1.2: 1 to feed intake with the mol ratio of tetramethyldipropylene triamine and dodecanoic acid, N 2 Protected, heated to 80°C under oil bath conditions, the material was completely melted and kept for 2.5 hours. Raise the temperature to 120°C and keep it warm for 2 hours, then continue to raise the temperature, the temperature gradually rises from 120°C to 160°C, the water generated during the reaction is continuously distilled out, and after 6 hours of reaction, the conversion rate of fatty acid reaches 70%. After the reaction is complete, transfer the crude reaction product to a separatory funnel, add 5% (w / w) sodium hydroxide solution, then add dichloromethane for extraction, let stand to separate layers, separate the dichloromethane layer, and wash with saturated salt Wash the dichloromethane layer with water for 3 times, dry the dichloromethane layer with anhydro...
Embodiment 2
[0030] Embodiment 2: the synthesis of double head group hexadecyl amido sultaine
[0031] According to the synthesis method in Example 1, the dodecanoic acid is replaced by hexadecanoic acid to prepare the intermediate N , N - Bis-(dimethylaminopropyl)hexadecylamide. Press propane sultone and intermediates N , N - The molar ratio of bis-(dimethylaminopropyl)hexadecylamide was 2.5:1 for feeding. First use acetone as solvent to dissolve the intermediate N , N -Bis-(dimethylaminopropyl)hexadecylamide, heat up to 60°C, after the solvent is stably refluxed, slowly add propane sultone dropwise through the dropping funnel, the reaction time is 48 hours, and suction filter after the reaction is completed Remove solvent acetone with N ,N - Dissolve the filter cake in dimethylformamide, recrystallize twice, and dry in vacuo for 48 hours to obtain a light yellow solid. intermediate N , N - Bis-(dimethylaminopropyl)hexadecylamide conversion >90%.
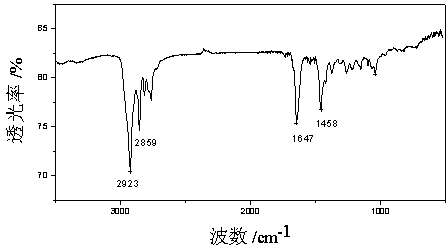
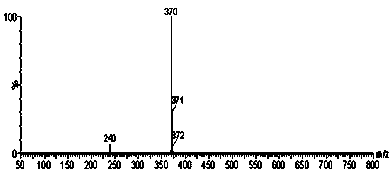
[0032] The purified product w...
Embodiment 3
[0033] Embodiment 3: the synthesis of double head group dodecyl amido carboxyl betaine
[0034] According to the synthesis method in Example 1, prepare N , N - bis-(dimethylaminopropyl) laurylamide. It is dissolved in ethanol, and sodium chloroacetate is dissolved in water for feeding, wherein N , N - The molar ratio of bis-(dimethylaminopropyl)dodecylamide to sodium chloroacetate is 1:3, and the volume ratio of ethanol to water is 5:1. The reaction temperature is 78-85°C, the reactants are homogeneous, and the reaction time is 8h. After the reaction is finished, the ethanol and water are removed by rotary evaporation, then absolute ethanol is added, the insoluble unreacted raw material sodium chloroacetate and the sodium chloride produced by the reaction are filtered off, and the purified double head group is obtained after being washed 3 times with dichloromethane. Laurylamidocarboxybetaine.
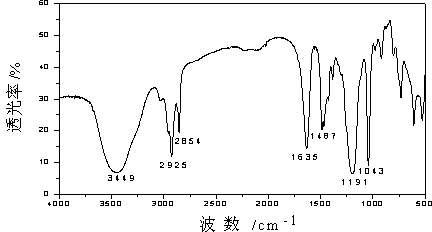
[0035] The purified product was analyzed by infrared spectroscopy, and the r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com