Radiation curable aqueous dispersions
A technology of radiation curing and dispersion, which is applied in the field of obtaining it, can solve the problems of undisclosed radiation curable composition, etc., and achieve the effect of long service life and good surface curing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
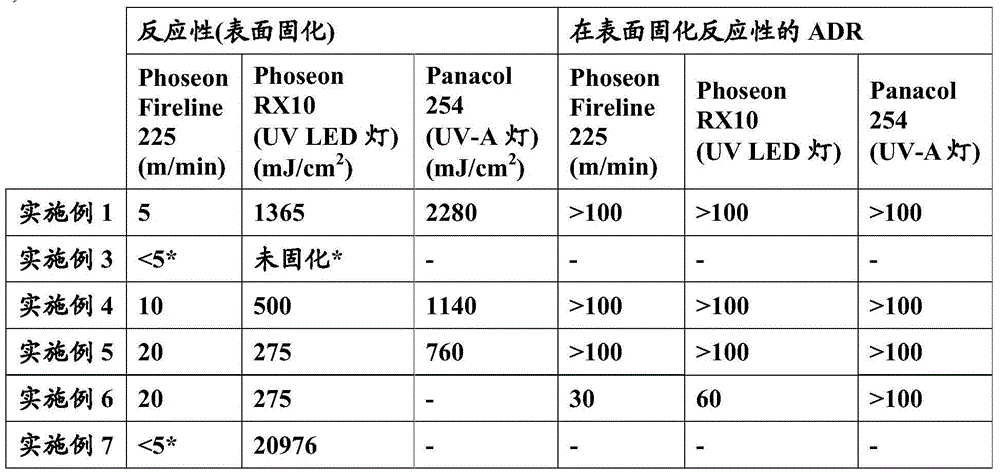
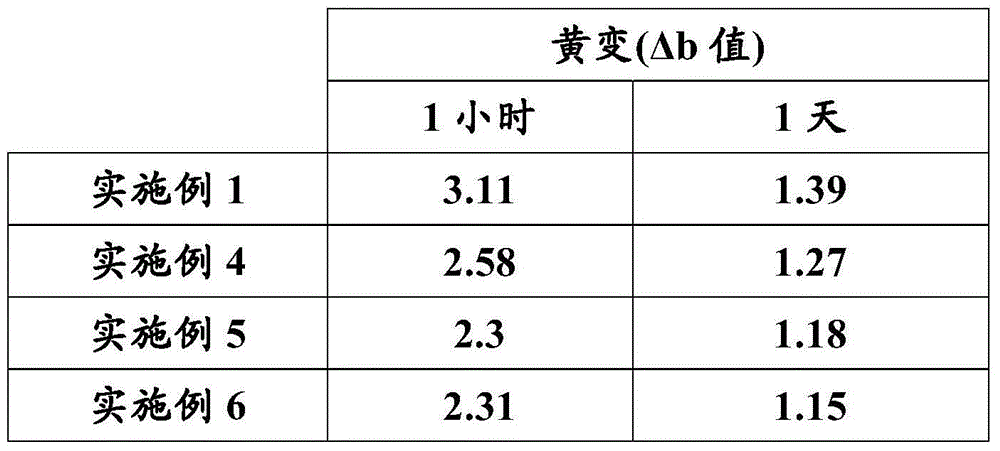
Examples
Embodiment 1
[0205] Example 1: Preparation of a dispersion comprising acrylated polyurethane prepolymer A1.
[0206] In a double-walled glass reactor equipped with a mechanical stirrer, a thermocouple, an evaporative condenser and a dropping funnel, 37.8 g of polyester polyol (which has an average molecular weight of 670, a hydroxyl value of 167 mgKOH / g, and was obtained from neopentyl glycolate polycondensation of alcohol with a mixture of adipic and isophthalic acids in a 1:1 weight ratio), 91 g of acrylic acid adduct of bisphenol A diglycidyl ether (BPAAA), 28.6 g of dimethylol propionic acid (DMPA), 192.7g of 1,1'-methylenebis(4-isocyanatocyclohexane) (H12MDI), 223g of acetone, 3.3g of 622 and 0.5g of dibutyltin laurate in 10wt% acetone solution. The reaction mixture was heated with stirring up to 600° C. and kept at reflux until the isocyanate content reached a value of 1.09 meg / g. 0.4 g of 4-methoxyphenol (dissolved in 319 g of a reaction mixture of dipentaerythritol tetraacrylat...
Embodiment 2
[0208] Example 2: Preparation of a dispersion comprising acrylated polyurethane prepolymer A2.
[0209] In the double-wall glass reactor equipped with mechanical stirrer, thermocouple, evaporative condenser and dropping funnel, add the polyester polyol of 170.42g (the about 670 Daltons of its average molecular weight, 167mg KOH / g of hydroxyl value, and polycondensation of adipic acid and neopentyl glycol), 47.72 g of dimethylolpropionic acid, 21.98 g of cyclohexanedimethanol, 319.88 g of 1,1'-methylenebis(4-isocyanate Cyclohexane) (H12MDI), 240 g of acetone and 0.8 g of dibutyltin laurate in 10%wt acetone. The reaction mixture was heated to reflux with stirring and kept at reflux until the isocyanate content reached a value of 1.47 meq / g. Then 0.51 g of 4-methoxyphenol (which is dissolved in 220.75 g of DTMPTA and has a hydroxyl value of 137 mg KOH / g) is slowly added to the reactor, DTMPTA is a compound containing di(trimethylol)propane triacrylate and di(trimethylol)propa...
Embodiment 3
[0211] Example 3: Preparation of a dispersion comprising mercapto-functional polyurethane B.
[0212] Into a double-walled glass reactor equipped with a mechanical stirrer, thermocouple, evaporative condenser, and dropping funnel was charged 88.6 g of polyester polyol with an average molecular weight of about 670 Daltons (via adipic acid and neopentyl glycol polycondensation), 18.08g of dimethylolpropionic acid, 15.81g of cyclohexanedimethanol, 177.47g of 1,1'-methylenebis(4-isocyanatocyclohexane) (H12MDI) , 100 g of acetone and 0.4 g of acetone solution (10 wt %) of dibutyltin laurate as a reaction catalyst. The reaction mixture was heated to reflux with stirring and the condensation process was maintained until the isocyanate content reached 1.47 meq / g. Thereafter the mixture was further diluted with 232 g of acetone. The polyurethane prepolymer was cooled to 55°C. 199.3 g of trimethylolpropane trimercaptopropionate (tmpmp) and 0.83 g of triethylamine were added to the ve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com