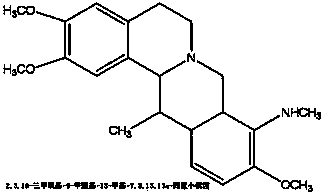
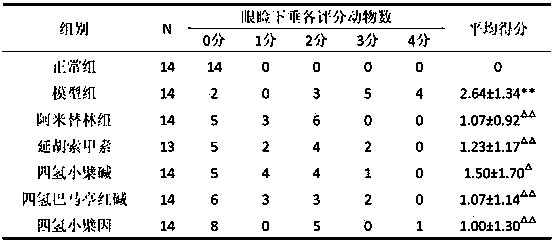
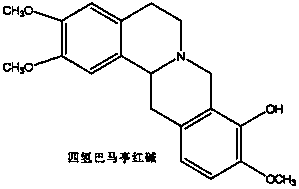
Application of tetrahydroproberberine compounds to prepare antidepressants
A technology of tetrahydroprotoberberine and compounds, which is applied in the field of tetrahydroprotoberberine compounds, and can solve the problem of not finding the antidepressant effect of tetrahydroprotoberberine compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0058] Extracting and Separating Corydalis A and Tetrahydroberberine from Corydalis Corydalis
[0059] 100 kg of dried tubers of Corydalis Corydalis were reflux extracted with 60% ethanol for 3 times, each time for 1.5 h, and the amount of solvent was 8 times. The extracts were combined and concentrated, and put on the treated pilot-scale D141 macroporous adsorption resin column, and purified water with 8 times the column volume to remove impurities such as sugar, amino acid, inorganic salt (Molish reaction negative, ninhydrin reaction negative), Elute with 8 times column volume of ethanol with a volume fraction of 90%, and concentrate to obtain 1.4 kg of total alkaloid fraction extract. Separation by silica gel column chromatography [(chromatographic solvent is ethyl acetate-dichloromethane-methanol-ammonia water (3:9:2.5:0.5)], combined with the color band under visible light and fluorescence under ultraviolet light, the silica gel And down cut into 15 sections.Be that the ...
preparation example 2
[0062] Semi-synthetic preparation of tetrahydroberberine
[0063] Add 15 g (0.045 mol) of berberine hydrochloride (berberine) and 300 ml of methanol into a 500 ml three-necked bottle, and slowly heat to reflux to dissolve all berberine. Add 4.5 g (0.085 mol) of potassium borohydride in batches, keep it warm for 1 h, and then react at room temperature for 3-4 h. A large amount of light yellow solid is produced, which is filtered off, washed with water, and dried to obtain 12.5 g of light yellow powder. Recrystallized from 95% ethanol to obtain 9.5 g of off-white crystals with a yield of 63%. Physicochemical and spectral data of tetrahydroberberine: white massive crystals (95%). ESI-MS m / z: 340[M+H ]+ . 1 H-NMR (CDCl 3 ) δ: 6.86 (1H, d, J = 8.4 Hz), 6.79 (1H, d, J = 8.4 Hz), 6.73 (1H, s), 6.59 (1H, s), 5.92 (2H, s, O-CH 2 -O), 4.24(1H, d, J = 15.6 Hz), 3.85(6H, s, 2×OCH 3 ), 3.38 (2H, m), 3.19 (3H, m), 2.82 (1H, m), 2.64 (2H, m). 13 C-NMR (CDCl 3 ) δ: 50.4 (C-9), 146.3, ...
preparation example 3
[0065] Semi-synthetic preparation of tetrahydropalmatine
[0066] 11.2 g (2.9×10 -2 mol) dried palmatine hydrochloride was placed in a vacuum oven, heated to 190°C, and reacted for 7 h under a pressure of 10-15 mmHg to obtain a dark red powder. The crude product was prepared with chloroform / methanol (V / V = 8:1) Eluted by column chromatography, the bright red solid powder palmatine red base (5.7 g, 1.7 × 10 -2 mol, yield 59%). Spectroscopy data of palmatine: 1 H-NMR (DMSO- d 6 , 500 MHz): δ 9.09(1H, s, H-8), 8.05(1H, s, H-13), 7.50(1H, s, H-1), 7.21(1H, d, J=8.0 Hz, H-11), 6.96(1H, s, H-4), 6.38(1H, d, J=8.0 Hz, H-12), 4.49(2H, t, J=6.0 Hz, H-6), 3.88 ( 3H,s,-OCH 3 ), 3.81 (3H, s, -OCH 3 ), 3.72 (3H, s, -OCH 3 ), 3.05 (2H, t, J=6.0 Hz, H-5). Weigh palmatine red base (raw material) 5 g (1.5 × 10 -2 mol), placed in a 500 mL round-bottomed flask, successively added 1.5 g of sodium borohydride, 200 mL of 95% ethanol, magnetically stirred, heated and refluxed for 18 h, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com