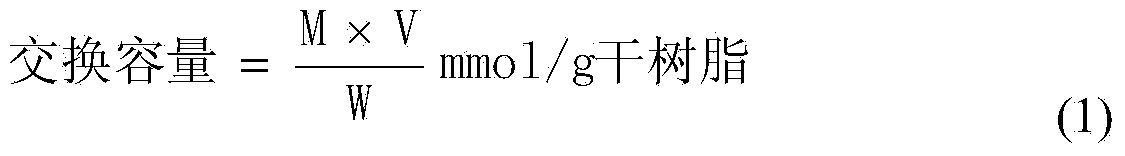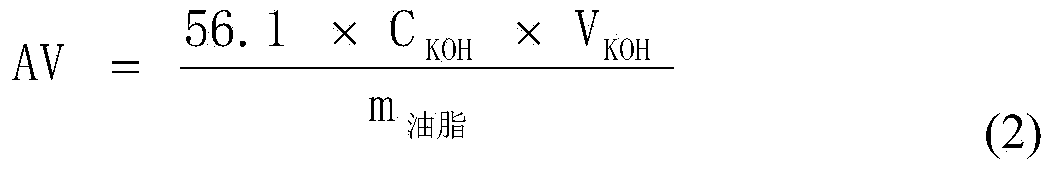Preparation method of sulfoacid-type cation exchange resin catalyst
A cation exchange and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, fatty acid esterification, etc., can solve the problems of complex process, expensive, strong corrosion, etc., and achieve efficient catalytic esterification reaction , simple preparation process and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Weigh 2.0 g of dry macroporous cross-linked polystyrene-divinylbenzene white balls into a three-necked flask equipped with a stirring and reflux condenser, add 10.0 g of dichloroethane, and swell at 20°C for 2 hours. Add 20.0 g of methanesulfonic acid, and start stirring at a slow speed. Raise the temperature to 85°C and keep it warm for 6.0h; raise the temperature to 100°C and keep it warm for 3.0h to remove dichloroethane by distillation. Cool to room temperature, add 40% methanesulfonic acid to wash, then slowly add deionized water dropwise under stirring, repeatedly wash until neutral, and vacuum dry at 60-120°C to obtain a macroporous sulfonic acid cation exchange resin catalyst.
[0013] Calculate the exchange capacity of the catalyst:
[0014]
[0015] in:
[0016] M—concentration of NaOH standard solution (mol / L);
[0017] V—the volume of NaOH standard solution used for titration (mL);
[0018] W—weight of dry resin (g).
[0019] Hydrothermal stability t...
Embodiment 2
[0028] Weigh 2.0 g of dry macroporous cross-linked polystyrene-divinylbenzene white balls, add 4.5 g of dichloroethane, and heat up to 45° C. to swell for 3.0 h. Add 10.0 g of methanesulfonic acid, and start stirring at a slow speed. Raise the temperature to 95°C and keep it warm for 3.0h; raise the temperature to 120°C and keep it warm for 2.0h to remove dichloroethane by distillation. Cool to room temperature, add 80% methanesulfonic acid to wash, then slowly add deionized water dropwise under stirring, repeatedly wash until neutral, and vacuum dry at 60-120°C to obtain a macroporous sulfonic acid cation exchange resin catalyst.
[0029] The exchange capacity of the obtained catalyst and the conversion rate of FFA when used in the esterification reaction are shown in Table 1.
Embodiment 3
[0033] Weigh 2.0 g of dry macroporous cross-linked polystyrene-divinyl benzene white balls into a three-necked flask equipped with a stirring and reflux condenser, add 1.0 g of dichloroethane, and heat up to 80°C to swell for 10 min. Add 40.0 g of methanesulfonic acid, and start stirring at a slow speed. Raise the temperature to 70°C and keep it warm for 1.0h; raise the temperature to 150°C and keep it warm for 0.5h to remove dichloroethane by distillation. Cool to room temperature, add diluted 70% methanesulfonic acid to wash, then slowly add deionized water dropwise under stirring, wash repeatedly until neutral, and vacuum dry at 60-120°C to obtain a macroporous sulfonic acid cation exchange resin catalyst .
[0034] Table 1 shows the exchange capacity of the catalyst and the conversion rate of FFA when used in the esterification reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com