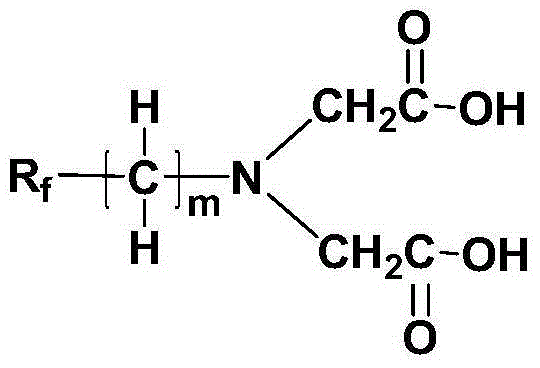
Fluorine-containing chelating agent and its preparation method and application
A chelating agent and reaction technology, applied in the field of fluorine-containing chelating agent and its preparation, can solve the problems of poor solvency, high operating pressure, low extraction efficiency, etc., achieve low extraction pressure, reduce energy consumption and operating costs, and improve extraction efficiency high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Taking the preparation of the fluorine-containing chelating agent of the following formula as an example, its specific preparation method is as follows:
[0027]
[0028] 1. Synthesis of diethyl iminodiacetate
[0029] Under nitrogen protection and condensing reflux, add 71.4g (0.6mol) of thionyl chloride dropwise into a round-bottomed flask containing 33.3g (0.25mol) of iminodiacetic acid, heat to 45°C under stirring, and react at a constant temperature After 5 hours, add 117mL (2mol) of ethanol, raise the temperature to 60°C and react at this temperature for 12 hours, after distilling off excess ethanol, then distill under reduced pressure to obtain 45.6g of diethyl iminodiacetate, the yield is 92%.
[0030] 2. Synthesis of fluorine-containing chelating agent
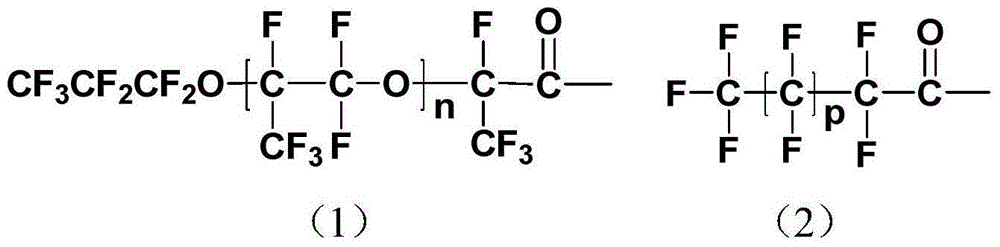
[0031] Under nitrogen protection and condensing reflux, 49.8g (0.10mol) hexafluoropropylene oxide trimer (CF 3 CF 2 CF 2 OCF (CF 3 ) CF 2 OCF (CF 3 ) COF, provided by Zhejiang Huanxin Fluorine Materi...
Embodiment 2
[0037] Taking the preparation of the fluorine-containing chelating agent of the following formula as an example, its specific preparation method is as follows:
[0038]
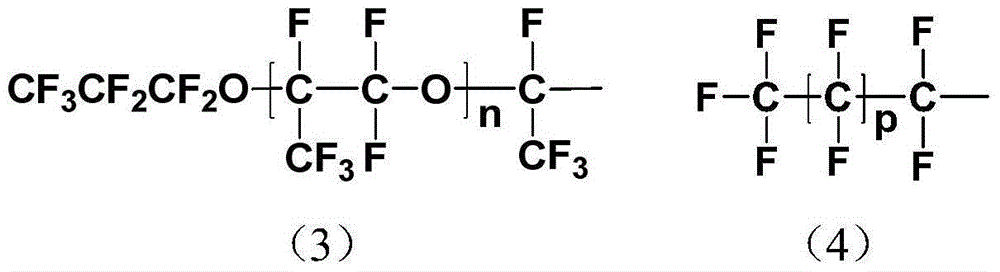
[0039] The synthesis of diethyl iminodiacetate in this example is the same as in Example 1. In step 2 of synthesizing a fluorine-containing chelating agent, 100 g (0.025 mol) of polyhexafluoro Propylene oxide (CF 3 CF 2 CF 2 O(CF(CF 3 ) CF 2 O) 22 CF(CF 3 ) COCl, the average degree of polymerization is 24, by the corresponding polyhexafluoropropylene oxide (CF) containing carboxylic acid end group 3 CF 2 CF 2 O(CF(CF 3 ) CF 2 O) 22 CF(CF 3 )COOH) and thionyl chloride were synthesized according to the method reported in the literature (J.Mater.Res., 1995,10,3,530-537), in which polyhexafluoropropylene oxide containing carboxylic acid end groups was provided by DuPont, USA) Put 5.68g (0.03mol) of diethyl iminodiacetate and 1.73g (0.0125mol) of anhydrous potassium carbonate into a round-bottomed ...
Embodiment 3
[0044] Taking the preparation of the fluorine-containing chelating agent of the following formula as an example, its specific preparation method is as follows:
[0045]
[0046] The synthesis of diethyl iminodiacetate in this example is the same as in Example 1. In step 2 of synthesizing a fluorine-containing chelating agent, under nitrogen protection and condensing reflux, 140 g (0.02 mol) of polyhexafluoro Propylene oxide (CF 3 CF 2 CF 2 O(CF(CF 3 ) CF 2 O) 40 CF(CF 3 ) COCl, the average degree of polymerization is 42, by the corresponding polyhexafluoropropylene oxide (CF) containing carboxylic acid end group 3 CF 2 CF 2 O(CF(CF 3 ) CF 2 O) 40 CF(CF 3 )COOH) and thionyl chloride were synthesized according to the method reported in the literature (J.Mater.Res., 1995,10,3,530-537), in which polyhexafluoropropylene oxide containing carboxylic acid end groups was provided by DuPont, USA) Put 4.75g (0.025mol) iminodiacetic acid diethyl ester and 2.1g (0.015mol) a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com