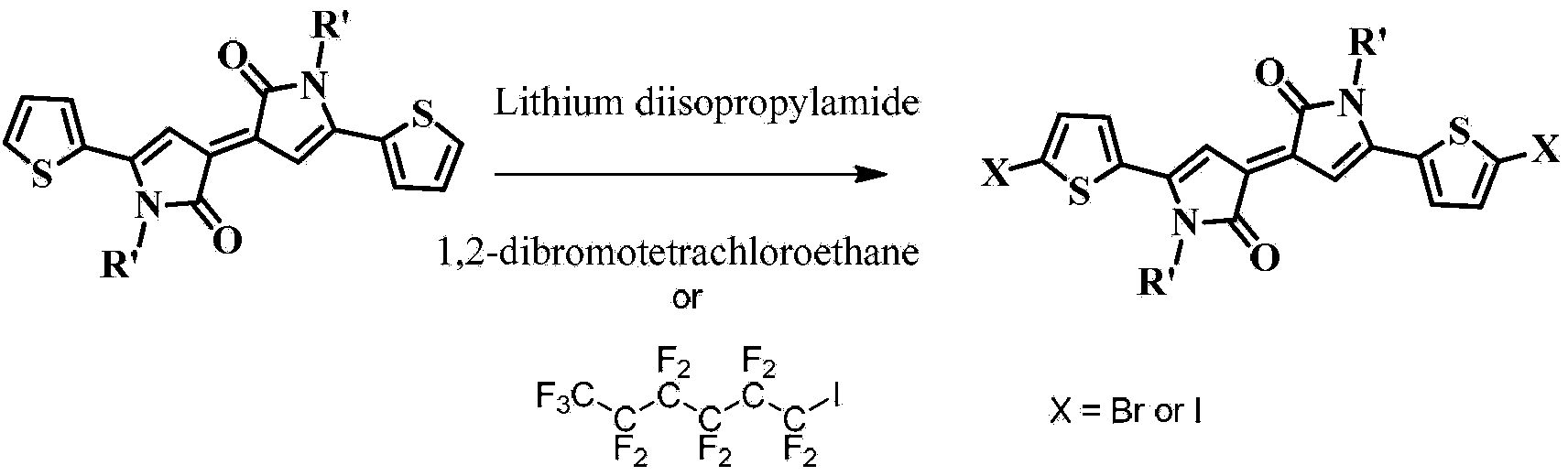Conjugated micromolecule material based on bithiophene dipyrrole and derivatives thereof, and preparation method and application thereof
An alkyl and aryl technology, applied in the field of conjugated small molecule materials, can solve the problems of reporting, no devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
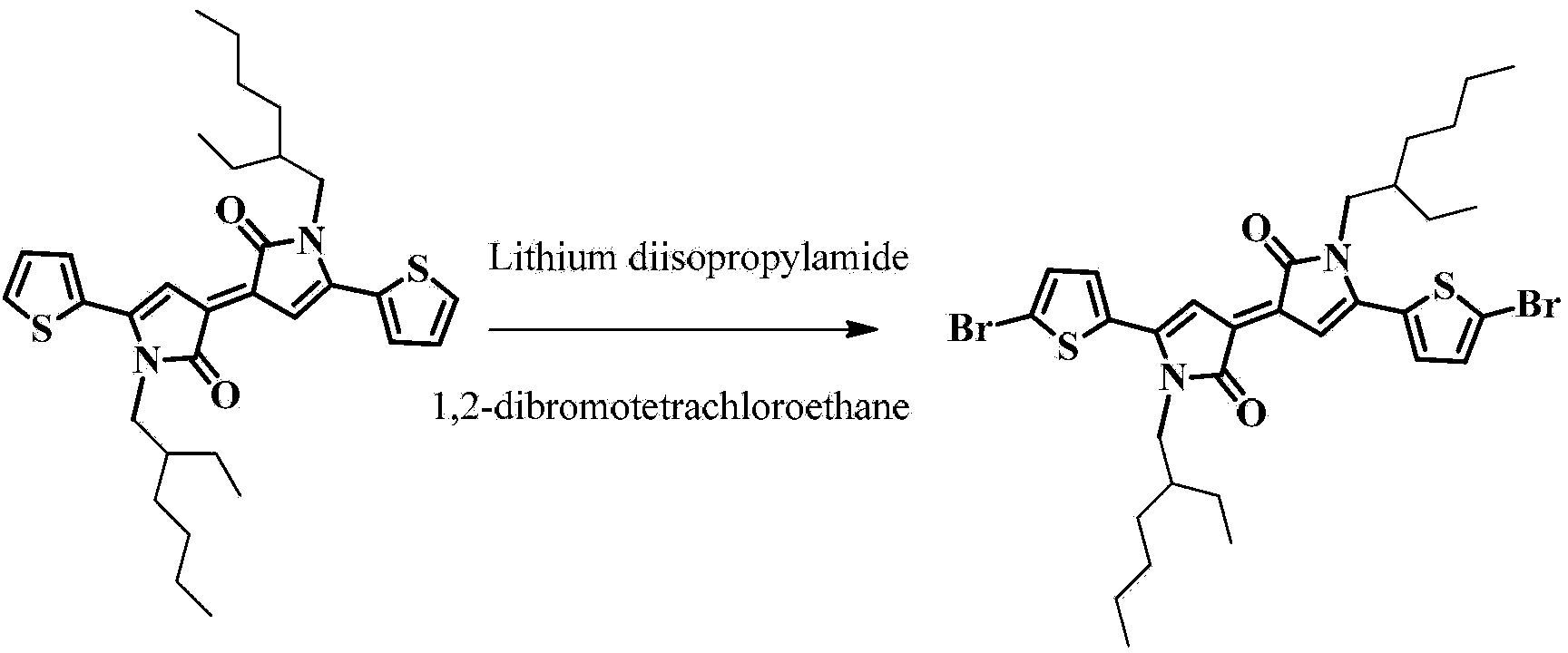
[0086] Embodiment 1: Synthesis of dibromobis (ethylhexyl) bithienylidene dipyrrole shown in formula II
[0087] chemical reaction flow chart image 3 As shown, the specific reaction conditions are as follows:
[0088] At low temperature (-78°C), in a nitrogen atmosphere, dissolve 0.89 mmol of bithiophenylidene dipyrrole (reported and synthesized by Tyler B. Norsten, published in the journal Chem. Eur. J. 2012, 18, 695-708) in 20 mL of tetrahydrofuran , add organic base lithium diisopropylamide (2.25mmol) for substitution reaction for 1 hour, then add 1,2-dibromotetrachloroethane (2.76mmol) for substitution reaction for 5 hours, then add water, use dichloro Methane was extracted three times, and the organic phases were combined and dried over anhydrous sodium sulfate. After removing the solvent, the product (0.41 mmol, 46%) was obtained by column separation with silica gel. The structural confirmation data are as follows: 1 H NMR (300MHz, CDCl 3 )δ7.17(d,2H,J=3.9Hz),7.08(d...
Embodiment 2
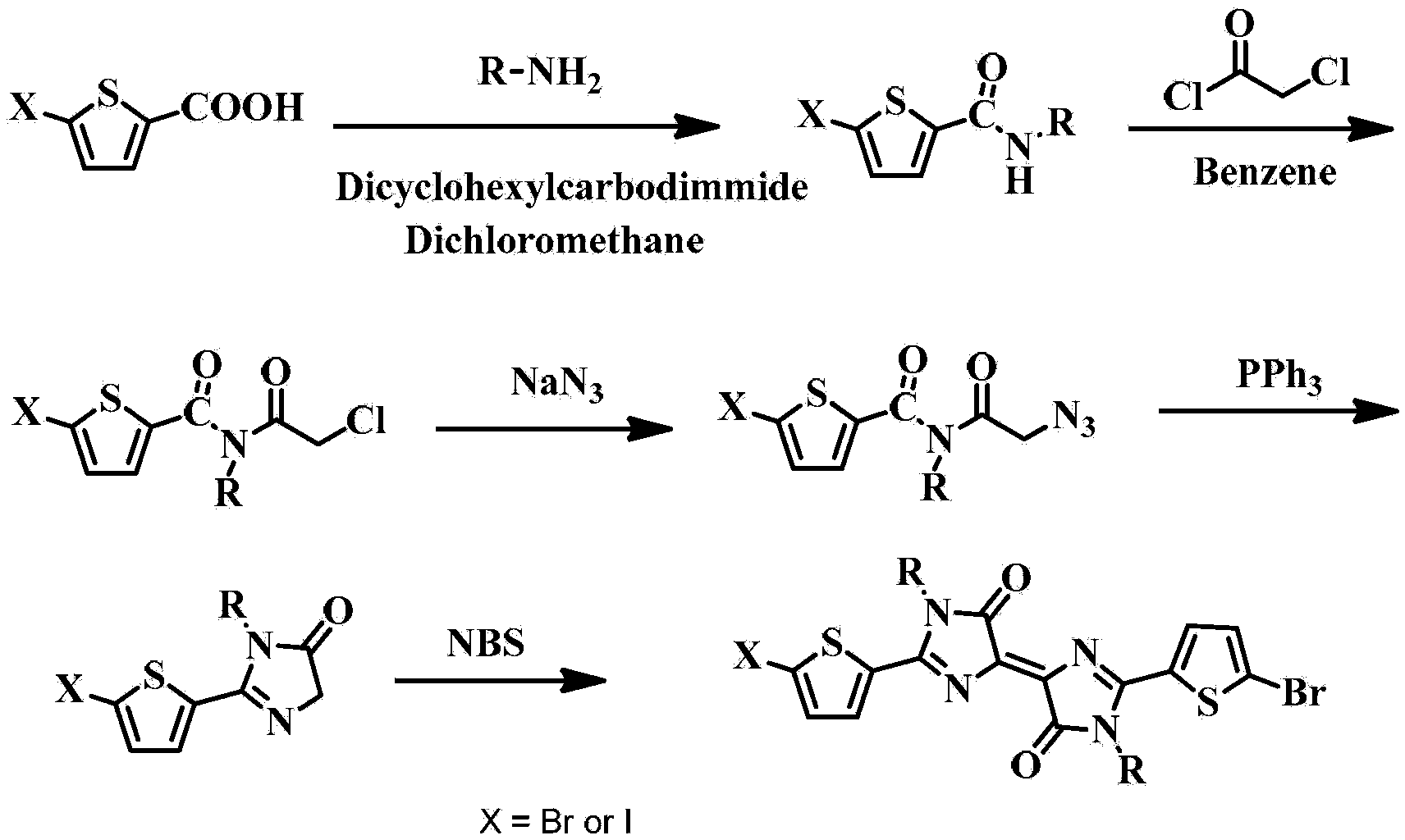
[0089] Embodiment 2: Synthesis of dibromobis(ethylhexyl)bithienylidene dipyrrole derivatives shown in formula III
[0090]chemical reaction flow chart Figure 4 Shown, concrete reaction step condition is as follows:
[0091] Step 1: At 0°C, dissolve 25 mmol of 5-bromothiophene 2-carboxylic acid (synthesized by Shih, Chuan, published in Journal of Medicinal Chemistry, 1992, 36, 1109-1116) in 50 mL of dichloromethane, add Active agent dicyclohexylcarbodiimide 30mmol, then add R-NH 2 (R is ethylhexyl) 0.275mmol, carried out amidation reaction at room temperature for 5 hours, filtered, washed with hydrochloric acid and potassium carbonate solution, dried with anhydrous sodium sulfate, and separated by silica gel column to obtain the product (16.7mmol 70% ). The structural confirmation data are as follows: elemental analysis: the calculated value is C 13 h 20 BrNOS: C, 49.06; H, 6.33; N, 4.40; S, 10.07; actual value: C, 49.26; H, 6.36; N, 4.20; S, 10.28.
[0092] Step 2: At r...
Embodiment 3
[0097] Example 3: Synthesis of dibenzofuran bis(ethylhexyl)bithienylidene dipyrrole shown in formula I
[0098] chemical reaction flow chart Figure 5 As shown, the specific reaction conditions are as follows:
[0099] At room temperature, the product synthesized in Example 1 (0.14mmol) was dissolved in 50mL of tetrahydrofuran, then 2(tributyltin)benzofuran (0.36mmoL) was added, and tetrakis(triphenylphosphine)palladium 0.007mmol was added, and heated at 60° C was stirred for 10 hours, the reaction was stopped, and the product (0.12 mmol 89%) was obtained by separation with a silica gel column after removing the solvent. The structural confirmation data are as follows: 1 H NMR (300MHz, CDCl 3 )δ7.59(d,2H,J=7.5Hz),7.53-7.49(m,6H),7.32-7.28(m,4H),7.23(m,2H),6.97(s,2H),3.89-3.87 (m, 4H), 1.72(m, 2H), 1.35-1.26(m, 16H), 0.89-0.84(m, 12H); elemental analysis: the calculated value is C 48 h 50 N 2 o 4 S 2 :C,73.62;H,6.44;N,3.58;S,8.19;actual value:C,73.44;H,6.43;N,3.56;S,8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com