Li and Mn codoped manganese phosphate/carbon composite material and preparation method thereof
A carbon composite material, lithium manganese phosphate technology, applied in electrical components, battery electrodes, circuits, etc., can solve the problems of difficult control of particle size and distribution, low discharge specific capacity, unstable cycle, etc., and achieve the suppression of particle agglomeration , speed up the diffusion rate, increase the effect of the reaction contact area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
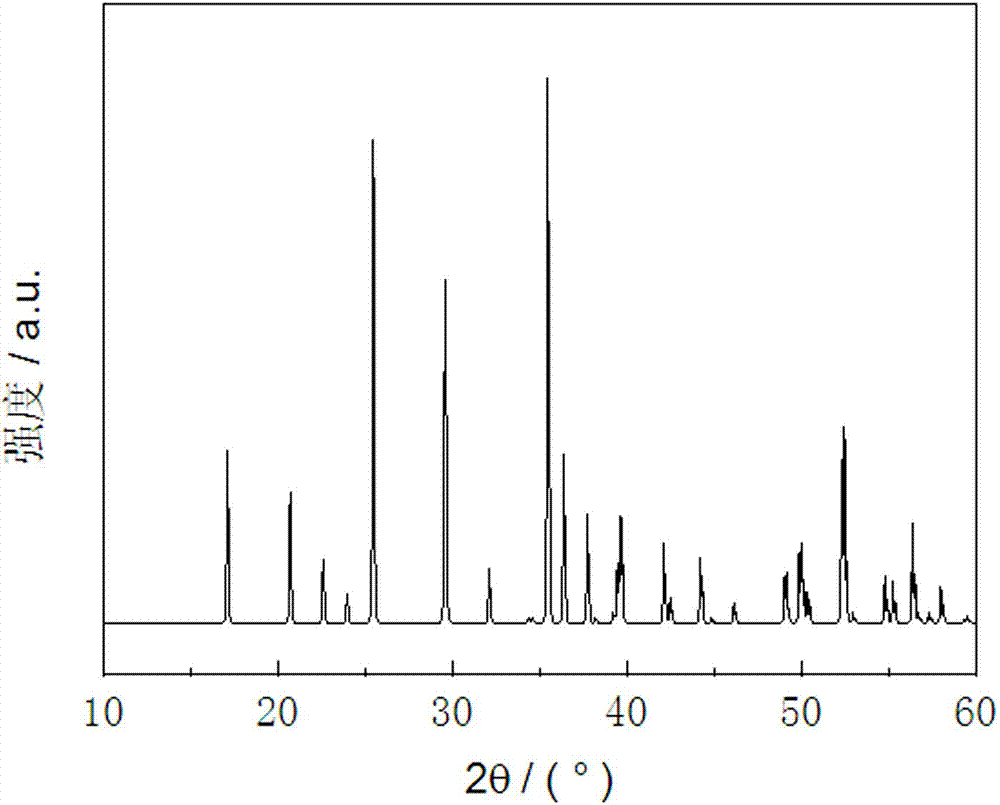
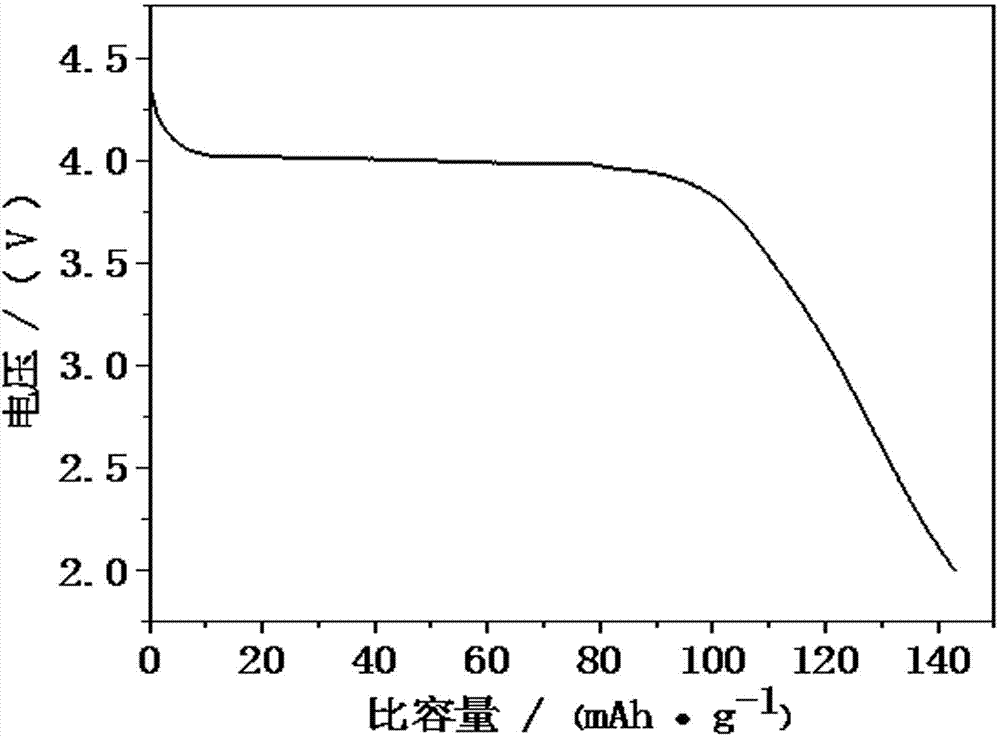
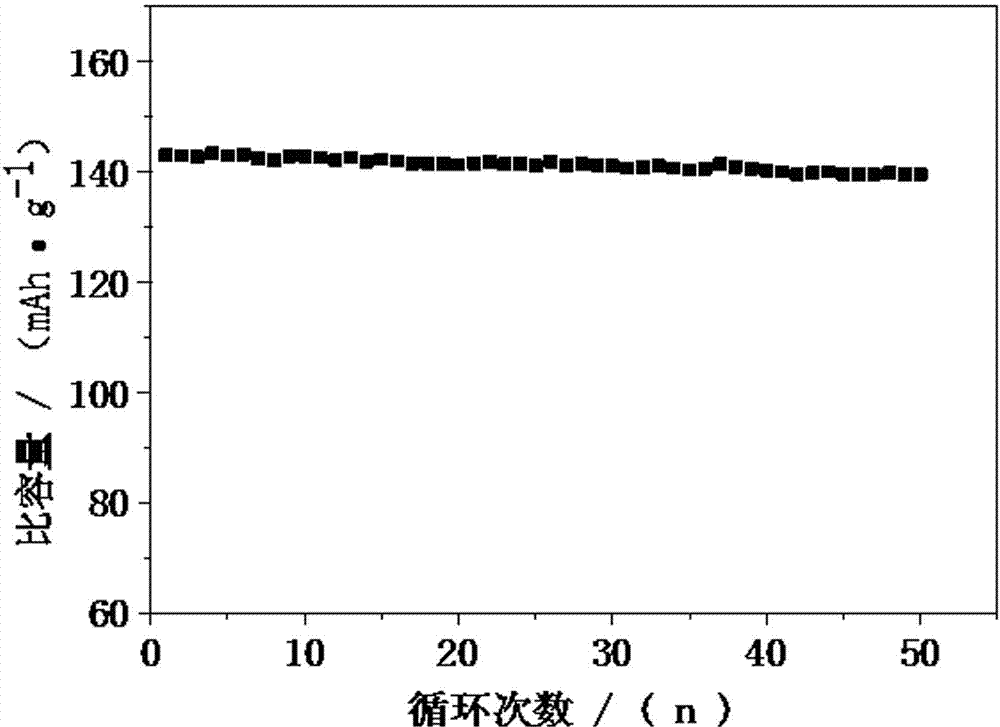
Image
Examples
Embodiment 1
[0030] Disperse manganese carbonate and ferrous oxalate in a 30.0wt% oxalic acid solution at a molar ratio Mn:Fe of 0.88:0.12, and stir for 0.5 hours to form a 2.0mol / L emulsion. The emulsion was flash-dried to obtain a dry mixed powder, and the mixed powder was calcined at 600 °C for 2 hours under the protection of argon to synthesize the product Mn 0.88 Fe 0.12 O, obtained nanoscale Mn by ball milling 0.88 Fe 0.12 O. Next, diammonium hydrogen phosphate, lithium hydroxide, magnesium hydroxide and nanoscale Mn 0.88 Fe 0.12 O is dispersed in the ratio of 1.04:0.9:0.12:0.88 by molar ratio P:Li:Mg:Mn in the oxalic acid solution of 30.0wt% and adds sucrose, and the addition amount of sucrose is ammonium dihydrogen phosphate, lithium hydroxide, magnesium hydroxide and Nanoscale Mn 0.88 Fe 0.12 20.0wt% of the total mass of the mixture formed by O was stirred for 3 hours to form a 2.0mol / L emulsion, and the emulsion was dried at 80°C for 8 hours to obtain a paste, which was st...
Embodiment 2
[0032] Disperse manganese oxalate and nickel acetate in a 30.0wt% oxalic acid solution at a molar ratio Mn:Ni of 0.92:0.08, and stir for 4 hours to form a 2.5mol / L emulsion. The emulsion was flash-dried to obtain a dry mixed powder, and the mixed powder was calcined at 550°C for 4 hours under the protection of nitrogen to synthesize
[0033] Product Mn 0.92 Ni 0.08 O, obtained nanoscale Mn by ball milling 0.92 Ni 0.08 O. Next, ammonium dihydrogen phosphate, lithium acetate, magnesium acetate and nanoscale Mn 0.92 Ni 0.08 O is dispersed in the ratio of 1.02:0.94:0.08:0.92 by molar ratio P:Li:Mg:Mn in 30.0wt% oxalic acid solution and adds citric acid, and the addition amount of citric acid is ammonium dihydrogen phosphate, lithium acetate, magnesium acetate, Nanoscale Mn 0.92 Ni 0.08 10.0wt% of the total mass of the mixture formed by O, stirred for 4 hours to form a 2.5mol / L emulsion, the emulsion was dried at 80°C for 6 hours to obtain a paste, and the paste was roasted...
Embodiment 3
[0035] Disperse manganese acetate and nickel hydroxide in a 50vol% ethanol solution at a molar ratio Mn:Ni of 0.85:0.15, and stir for 6 hours to form a 1.0mol / L emulsion. The emulsion was flash-dried to obtain a dry mixed powder, and the mixed powder was calcined at 400°C for 3 hours under the protection of argon to synthesize the product Mn 0.85 Ni 0.15 O, obtained nanoscale Mn by ball milling 0.85 Ni 0.15 O. Next, disodium hydrogen phosphate, lithium oxalate, copper acetate and nanoscale Mn 0.85 Ni 0.15 O is dispersed in a 50.0vol% ethanol solution with a molar ratio of P:Li:Cu:Mn of 1.03:0.87:0.15:0.85 and glucose is added. The amount of glucose added is disodium hydrogen phosphate, lithium oxalate, copper acetate, nano-scale mn 0.85 Ni 0.15 15.0wt% of the total mass of the mixture formed by O was stirred for 0.5 hours to form a 1.0mol / L emulsion, which was dried at 80°C for 2 hours to obtain a paste, which was stored at 750°C under the protection of argon Roast for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com