New preparation method of S-pantoprazole sodium
A technology of pantoprazole sodium and sodium bismuthate, which is applied in the field of medicine, can solve the problems of unfavorable industrial production, low chiral selectivity, and low utilization rate of raw materials, and achieve the advantages of convenient industrial production, high yield, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
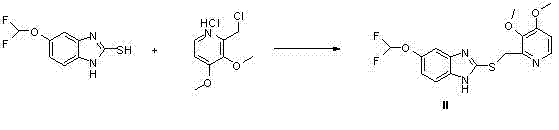
[0021] 1) Add 2 kg of 5-difluoromethoxy-2-mercapto-1H-benzimidazole into 20L aqueous solution of 900 g NaOH in a 50L reactor. Control the temperature at 20-25°C, add dropwise 7 L of aqueous solution of 2 kg 2-chloromethyl-3,4-dimethoxypyridine hydrochloride, control the temperature at 30°C, and keep stirring for 4 hours. Add 10 L of dichloromethane to the reaction kettle for extraction, separate the layers, extract the aqueous layer once with 10 L of dichloromethane, combine the organic phases, and wash with 10 L×2 water. The dichloromethane layer was dried by adding anhydrous sodium sulfate. After filtration, the filtrate was concentrated to obtain 3.21 kg of light yellow solid powder Compound II, with a yield of 94.41% and an HPLC purity of 99.24%.
[0022] 2) Add 20 L of toluene, 2.5 kg of compound II, 1.7 kg of N,N'-bis(3-pyridylmethyl)-D(-)-tartrate diamide, 1.0 kg of isopropyl titanate into a 50 L reactor , 850 mL of triethylamine, stirring the system to raise the temp...
Embodiment 2
[0025] 1) Add 1.89 kg 5-difluoromethoxy-2-mercapto-1H-benzimidazole to 20L aqueous solution of 875 g NaOH in a 50L reactor; control the temperature at 20-25 ℃, add dropwise 1.9 kg 2-chloroform Base-3,4-dimethoxypyridine hydrochloride 7 L aqueous solution, control the temperature at 40 ° C, keep stirring and react for 4 hours; add 10 L dichloromethane to the reaction kettle for extraction, separate the layers, and use 10 L Extract once with dichloromethane, combine the organic phases, and wash with 10 L × 2 water. The dichloromethane layer was dried by adding anhydrous sodium sulfate. After filtration, the filtrate was concentrated to obtain 2.8 kg of light yellow solid powder Compound II, with a yield of 90.32% and a HPLC purity of 99.30%.
[0026] 2) Add 20 L of toluene, 2.5 kg of compound II, 1.7 kg of N,N'-bis(3-pyridylmethyl)-D(-)-tartrate diamide, 1.0 kg of isopropyl titanate into a 50 L reactor , 850 mL of triethylamine, and the system was stirred and heated to 60°C. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com