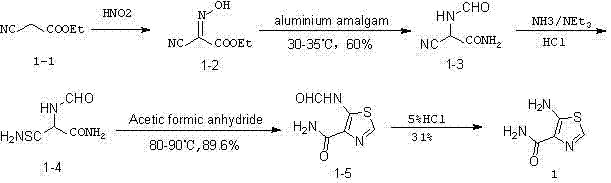
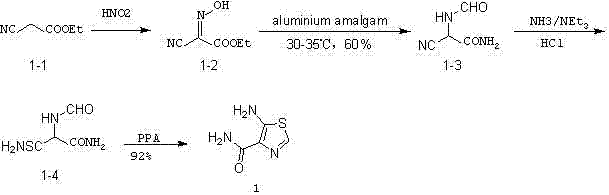
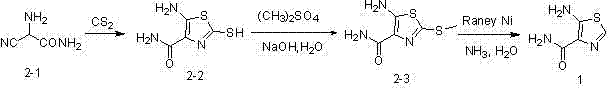Preparation method of 5-aminothiazole-4-formamide
A technology of aminothiazole and formamide, which is applied in the field of preparation of 5-aminothiazole-4-carboxamide and the synthesis of intermediates, can solve the problems of harsh reaction conditions, low yield, easy deliquescence of formic acetic anhydride, etc., and achieve mild reaction conditions , simple reaction process, simple post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Compound ( 2-2 )Synthesis
[0034]
[0035] In a 250ml four-neck bottle, add the compound ( 2-1 ) 2-Amino-2 cyanoacetamide (6.5g, 65.6mmol) and 70ml methanol were added 12ml (0.199mol) carbon disulfide, the reaction mixture was heated and refluxed for 1 hour, a large amount of solid precipitated out, cooled to 5℃, filtered with suction, The solid was washed with EA and dried to obtain the compound ( 2-2 ) Light yellow solid 10.9g, yield 94.8%.
[0036] Compound ( 2-3 )Synthesis
[0037]
[0038] In a 100ml three-necked flask, add the product compound of the previous step ( 2-2 ) (3.5g, 20mmol), 25ml of water and sodium hydroxide (0.88g, 22mmol). After stirring uniformly, it was cooled in an ice bath, and 2.1 ml of dimethyl sulfate (22 mmol) was added dropwise. Then react at room temperature for 1 hour, TLC shows that the reaction is complete. Cool to 0-5°C, filter with suction, wash the solid with a small amount of water, and dry to obtain 3.6 g of a yellow solid product wi...
Embodiment 2
[0043] Compound ( 2-2 )Synthesis
[0044]
[0045] In a 250ml four-necked flask, add 4.8ml (0.0798mol) carbon disulfide and 100ml ethyl acetate, cool to 0 °C, batch compound ( 2-1 ) 2-Amino-2 cyanoacetamide (5.5g, 54mmol), remove the ice bath, react for 1 hour, basically no reaction. Add 5.2ml (0.0865mol) carbon disulfide and 20ml methanol. The reaction mixture was heated and refluxed for 1 hour. A large amount of solid precipitated. Cooled to 5°C, filtered with suction, washed with EA, and dried to obtain 7.8g of light yellow solid. The rate is 80%.
[0046] Compound ( 2-3 )Synthesis
[0047]
[0048] In a 150ml three-necked flask, add the product compound of the previous step ( 2-2 ) (7.0g, 40mmol), 50ml water and sodium hydroxide (1.76g, 44mmol). Stir well and cool with an ice bath. When the temperature is 0℃-5℃, add 4.2ml of dimethyl sulfate (44mmol) dropwise. Then it was reacted at room temperature for 1 hour, TLC showed that the reaction was not complete. Then sodium hydrox...
Embodiment 3
[0053] Compound ( 2-2 )Synthesis
[0054]
[0055] In a 250ml four-neck bottle, add the compound ( 2-1 ) (19.5g, 196.8mmol) and 200ml of methanol, add 36ml (0.598mol) of carbon disulfide, the reaction mixture is heated to reflux for 30 minutes, a large amount of solid precipitates, cooled to 5 °C, suction filtered, the solid was washed with methanol, and dried to obtain Light yellow solid 26.7g, TLC has fluorescence. The mother liquor was left overnight, and another solid was precipitated, and 3.0 g of yellow solid was obtained by suction filtration. The total yield is 86.1%
[0056] Compound ( 2-3 )Synthesis
[0057]
[0058] In a 150ml four-neck bottle, add the product compound of the previous step ( 2-2 ) (3.5g, 20mmol), 25ml of water and sodium carbonate (2.4g, 22mmol). Stir well and cool with an ice bath. When the temperature drops to 0-5°C, add 2.1ml of dimethyl sulfate (22mmol) dropwise. After the addition is complete, the ice bath is removed, and the reaction is performed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com