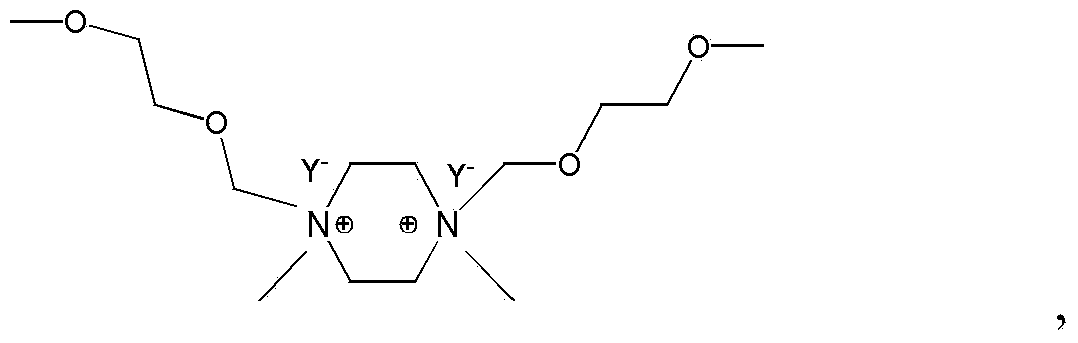
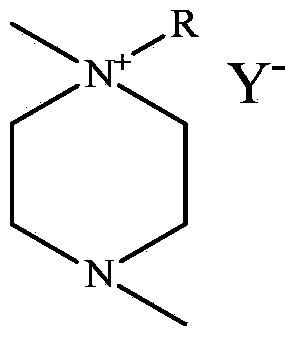
1, 4-dimethyl-1-alkyl piperazine ionic liquid as well as preparation method and applications thereof
A technology of alkylpiperazine and ionic liquid, applied in the field of organic synthesis, can solve problems such as long preparation period, and achieve the effects of short preparation period, high yield and low viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
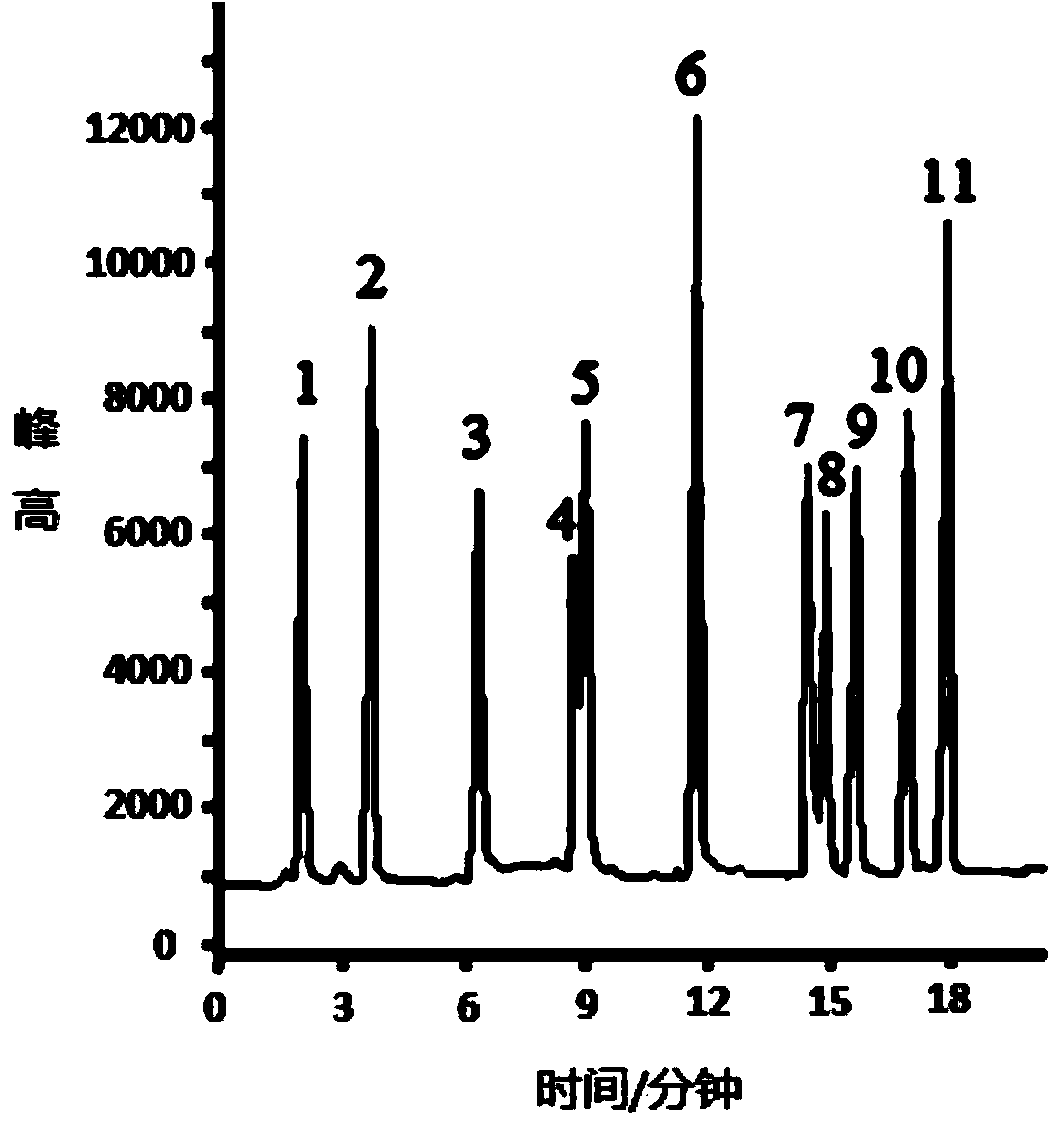
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of 1,4-dimethyl-1-ethylpiperazine ionic liquid chloride
[0034] In a 500mL three-necked flask equipped with a dropping funnel, reflux condenser and nitrogen balloon, add 1.3mol 1-chloroethane and 100mL ethyl acetate, and add 1mol 1,4-dimethylpiperazine and 100mL to the dropping funnel The ethyl acetate was slowly dropped into the ethyl acetate solution of 1-chloroethane, stirred and reacted at 80°C for 48 hours, cooled to room temperature, filtered, and the white solid obtained was washed with ethyl acetate and dried under vacuum at 40°C. The 1,4-dimethyl-1-ethylpiperazine chloride ionic liquid was obtained with a purity of 99% and a yield of 80%.
Embodiment 2
[0035] Example 2: Preparation of 1,4-dimethyl-1-propylpiperazine iodide ionic liquid
[0036] In a 500mL three-necked flask equipped with a dropping funnel, reflux condenser and nitrogen balloon, add 1.2mol 1-iodopropane and 100mL ethyl acetate, and add 1mol 1,4-dimethylpiperazine and 100mL acetic acid to the dropping funnel The ethyl ester was slowly dropped into the ethyl acetate solution of 1-iodopropane, stirred and reacted at 30°C for 12 hours, cooled to room temperature, filtered, and the white solid obtained was washed with ethyl acetate and dried under vacuum at 40°C to obtain iodine 1,4-Dimethyl-1-propylpiperazine ionic liquid, purity 99%, yield 86%.
Embodiment 3
[0037] Example 3: Preparation of 1,4-dimethyl-1-butylpiperazine iodide ionic liquid
[0038] In a 500mL three-necked flask equipped with a dropping funnel, a reflux condenser and a nitrogen balloon, add 1.1mol 1-iodobutane and 100mL ethyl acetate, and add 1mol 1,4-dimethylpiperazine and 100mL to the dropping funnel The ethyl acetate was slowly dropped into the ethyl acetate solution of 1-iodobutane, stirred and reacted at 40°C for 24 hours, cooled to room temperature, filtered, and the white solid obtained was washed with ethyl acetate and dried under vacuum at 50°C The 1,4-dimethyl-1-butylpiperazine iodide ionic liquid was obtained with a purity of 99% and a yield of 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com