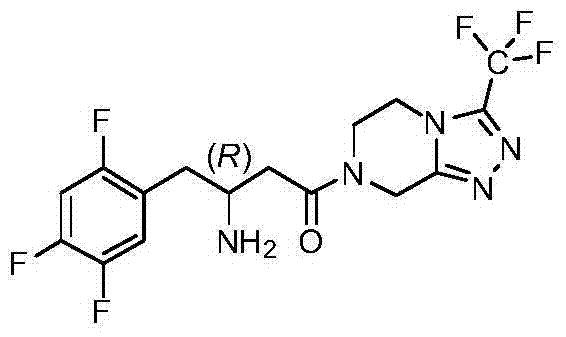
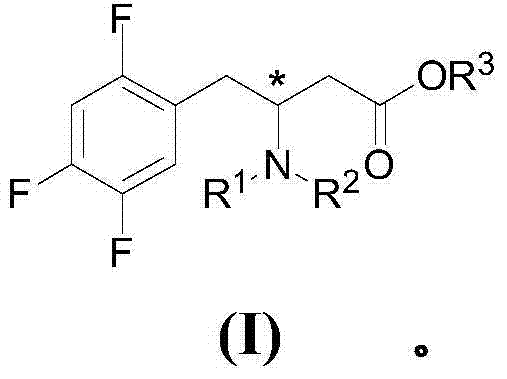
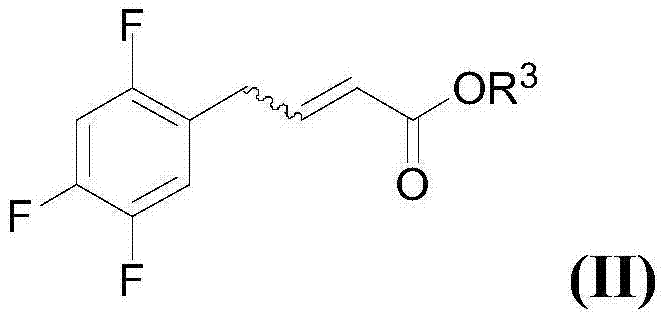
Preparation of sitagliptin intermediates
A technology of intermediates and chiral centers, applied in the field of preparation of chiral compounds, can solve problems such as toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0263] Example 1: Synthesis of (E)-4-(2,4,5-trifluorophenyl)-but-2-ene by cobalt-catalyzed cross-coupling process Acid methyl ester (IIa):
[0264] To a dry, nitrogen-flushed 200 mL two-neck flask equipped with a magnetic stirrer and rubber septum was added anhydrous THF (20 mL) and cooled to -20 °C. 2,4,5-Trifluorobenzene (65.2mmol, 13.7g, 7.6mL) was then introduced through the septum, followed by the very slow addition of iPrMgCl (2M in THF, 1.0 eq, relative to 2,4,5-trifluorobenzene Total, 39.6mL). The reaction temperature was maintained at -10°C and the reaction mixture was stirred for 1 hour until the Br / Mg exchange reaction was complete and 2,4,5-trifluoroarylmagnesium bromide (magnesium chloride) was formed.
[0265] Into another dry three-necked flask flushed with nitrogen, add cobalt(II) bromide (3.76mmol, 6mol%, relative to 2,4,5-trifluorobenzene, 822mg, 99.99% purity), TMEDA (3.76 mmol, 6mol%, relative to 2,4,5-trifluorobenzene, 564mL) and anhydrous THF (20mL)...
Embodiment 2
[0269] Example 2: Synthesis of 3-benzylamino-4-(2,4,5- from (IIa) in pure water by aza-Michael reaction Methyl trifluorophenyl)butyrate (Ib):
[0270] β-Unsaturated ester (E)-4-(2,4,5-trifluorophenyl)-but-2-enoic acid methyl ester (IIa) (0.5mmol, 115mg) and benzylamine (0.6mmol, 68 mg, 99% purity) was placed in a glass flask and deionized water (3 mL) was added. The heterogeneous reaction mixture (aqueous dispersion) was vigorously stirred (1000 rpm) at 60°C for 16 hours. The reaction mixture was diluted with water (5 mL) and extracted with EtOAc (2 x 25 mL). The combined organic layers were washed with anhydrous MgSO 4 After drying, the organics were evaporated under reduced pressure and the crude reaction mixture (165 mg) was obtained with 1 H NMR Spectroscopy. The crude product (Ib) was purified by column chromatography (SiO 2 , hexane:ethyl acetate=2:1) to obtain the light brown liquid product (Ib) (102mg, 61% yield). Compound 3-benzylamino-4-(2,4,5-trifluoroph...
Embodiment 3
[0273] Example 3: From (IIa) in Water by Aza-Michael Reaction in Phosphine Ligands and Surfactants Copper catalysis in the presence of [Cu(OAc) 2 ] Synthetic 3-benzylamino-4-(2,4,5-trifluorophenyl) methyl butyrate (Ib):
[0274] In a two-neck round bottom flask under nitrogen, add Cu(OAc) 2 (0.11mmol, 19.1mg), NatOBu (0.13mmol, 12.6mg), Ph 3 P (0.11 mmol, 28.8 mg) and anionic surfactant sodium dodecyl sulfate (SDS) (0.042 mmol, 12 mg). Deionized water (3 mL) was then added and the reaction mixture was vigorously stirred (900 rpm) at room temperature for 30 minutes. A 2 mL dispersion of β-unsaturated ester (E)-4-(2,4,5-trifluorophenyl)-but-2-enoate (IIa) (1 mmol) in deionized water was then passed through a rubber septum Slowly added to aqueous micellar solution (final volume: 5 mL of 0.0081 MSDS in water), followed by benzylamine (1 mmol, 107 mg, 110 μL). The aqueous reaction system was vigorously stirred (900 rpm) at room temperature for 6 hours. The reaction mixtu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com