Thermophilic alkaline recombination manganese-containing catalase as well as expression carrier and engineering bacteria thereof
A technology of manganese catalase and expression vector, applied in the fields of thermophilic alkaline recombinant manganese-containing catalase and its expression vector and engineering bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The construction of embodiment 1 manganese catalase MnCAT prokaryotic expression genetic engineering bacteria
[0062] 1.1 MnCAT gene designed based on codon usage preference optimization in Escherichia coli
[0063] According to the preference of codon usage in Escherichia coli ( http: / / gcua.schoedl.de / seqoverall_v2.html ) optimization design, the resulting codon-optimized MnCAT gene has a nucleotide sequence as shown in SEQ ID No:1.
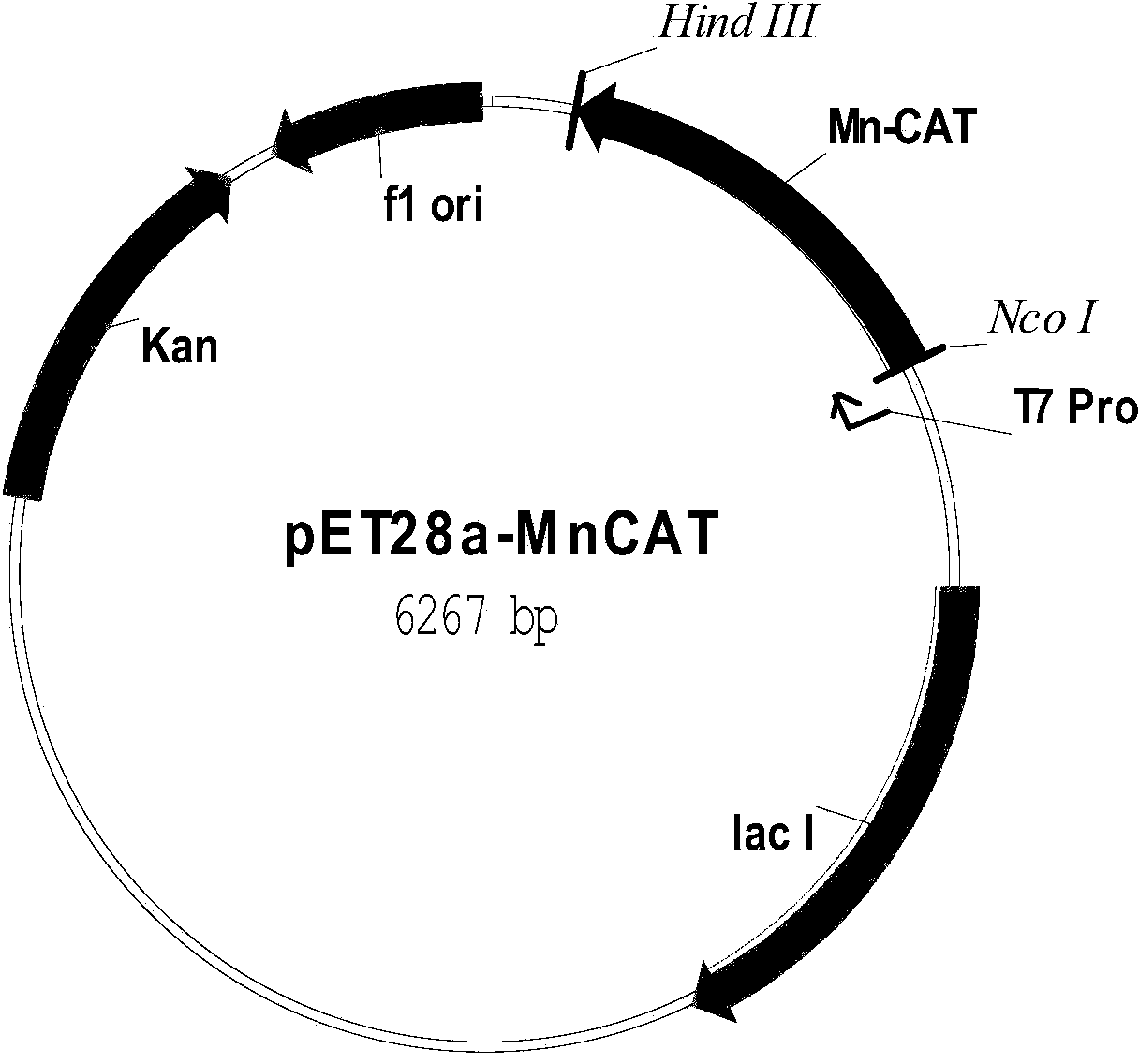
[0064] 1.2 Construction of MnCAT prokaryotic expression vector pET28a-MnCAT
[0065] At the 5'-end and 3'-end of the MnCAT gene, respectively design the restriction endonuclease Nco I and Hind III enzyme cutting sites that do not have in the gene but have on the multiple cloning site of the vector pET28a (+) (due to direct Adding the endonuclease Nco I nucleotide sequence CCATGG at the N-terminus of the gene will cause a frameshift mutation during the translation of the MnCAT gene. Therefore, in order to prevent the frameshift mutation...
Embodiment 2
[0068] Induced expression of embodiment 2 manganese catalase MnCAT
[0069] 2.1 Seed culture
[0070]Put the genetically engineered bacteria PB-01 into the LB medium containing 50μg / ml kanamycin, put 50ml of the medium into a 250ml culture bottle, at 37°C, the rotation speed is 200rpm, and the culture time is 10-12 hours. After cultivation, the OD600 absorbance value was between 4 and 5.
[0071] Note: The components of LB medium (g / L) are: 10 peptone, 5 yeast powder, and 10 sodium chloride.
[0072] 2.2 Shake flask fermentation culture:
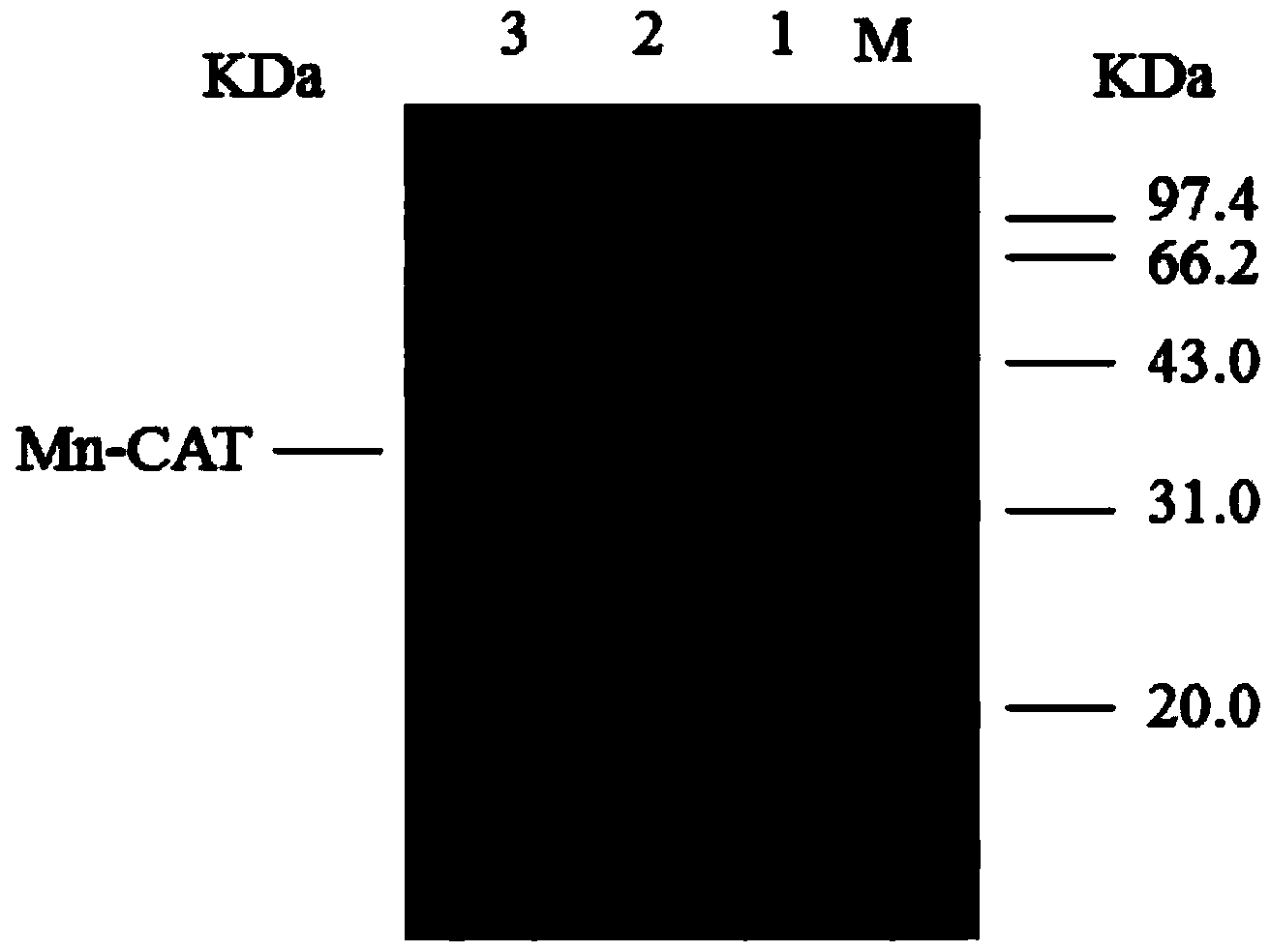
[0073] Transfer the seed culture solution to TB medium containing 50mg / L kanamycin (Kan) at 1%, when the recombinant bacteria E.coli PB-01 grows to OD 600 At 0.7-0.8, add a final concentration of 0.2mmol / L IPTG, and at the same time add a final concentration of 14mmol / L MnCl 2 , induced and cultured at 42°C for 2 hours, collected the bacteria, broken the cells, and analyzed the supernatant by SDS-PAGE. It was found that there was an obvi...
Embodiment 3
[0075] Example 3 Construction of Manganese Catalase MnCAT and Mnt H Co-expression Genetic Engineering Bacteria
[0076] 3.1 Construction of prokaryotic expression vector pACYCDuet-Mnt H of manganese ion transporter Mnt H
[0077] Using the genome of E.coli W3110 (E.coli Genetic Stock Center, Yale University) as a template, primers were designed to amplify the gene DNA fragment of manganese ion transporter Mnt H by PCR. The upstream primers used were:
[0078] P1: 5'- CATATG ACGAACTATCGCGTTGAGAGTAGC-3' (underlined Nde I restriction site).
[0079] Downstream primers are:
[0080] P2: 5'- CTCGAG CTACAAATCCCAGCGCCGTC-3' (underlined Xho I restriction site).
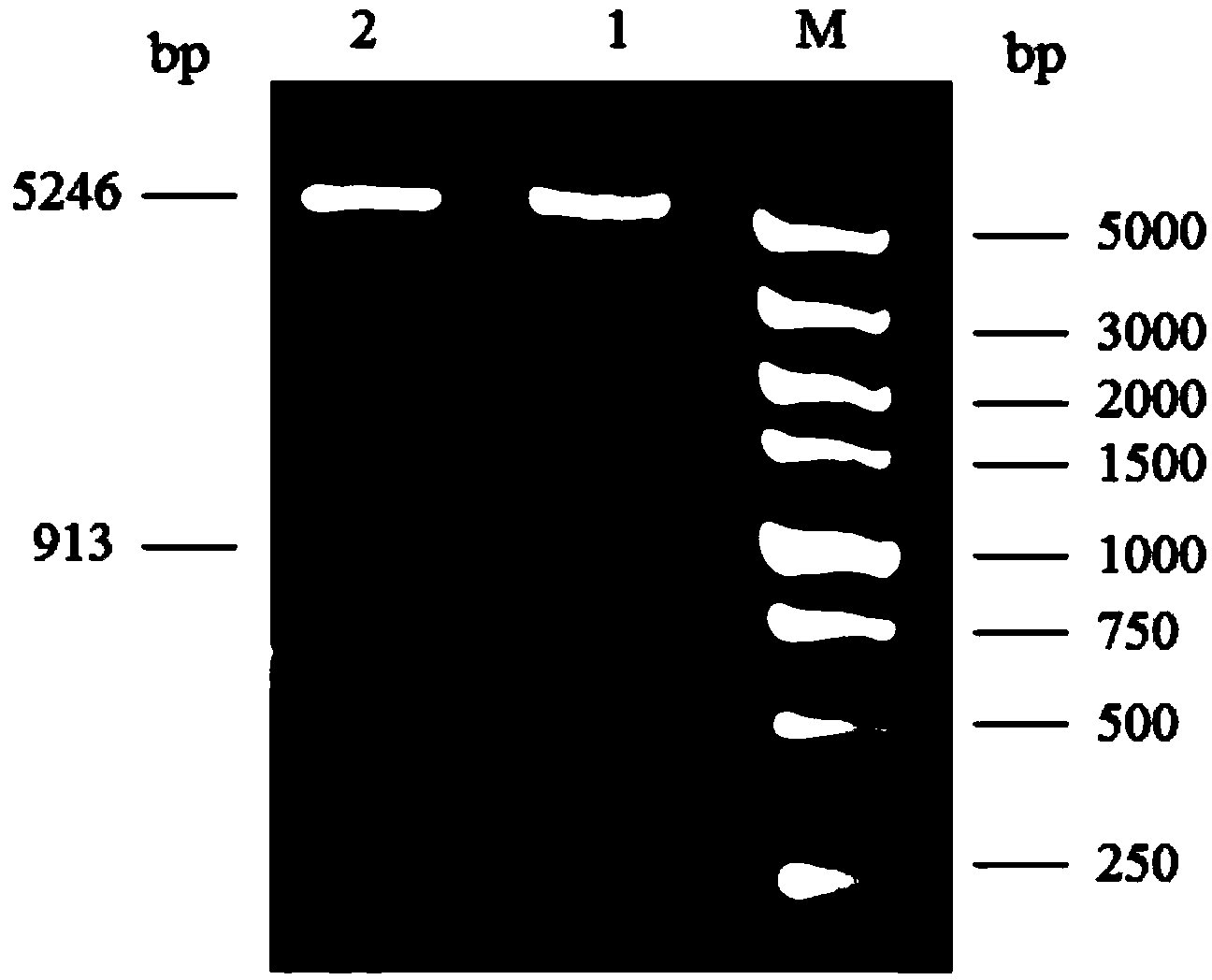
[0081] The PCR product was recovered by gel and digested with PstI / PvuII, and then cloned into the intracellular expression vector pACYCDuet-1 after the same digestion to construct the recombinant expression plasmid pACYCDuet-Mnt H, which was verified by sequencing and stored in Escherichia coli DH5α ( Figure 4 , ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com