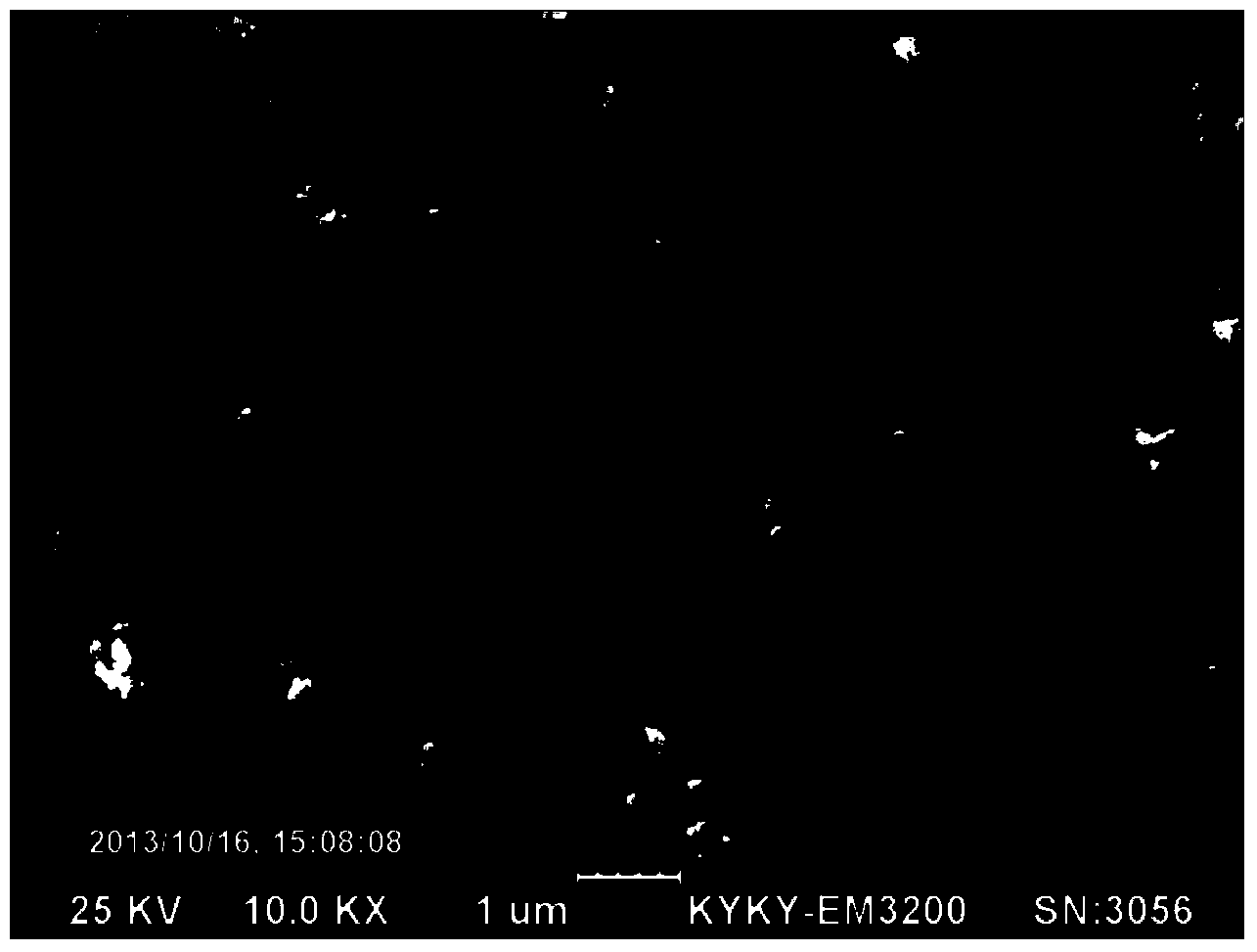
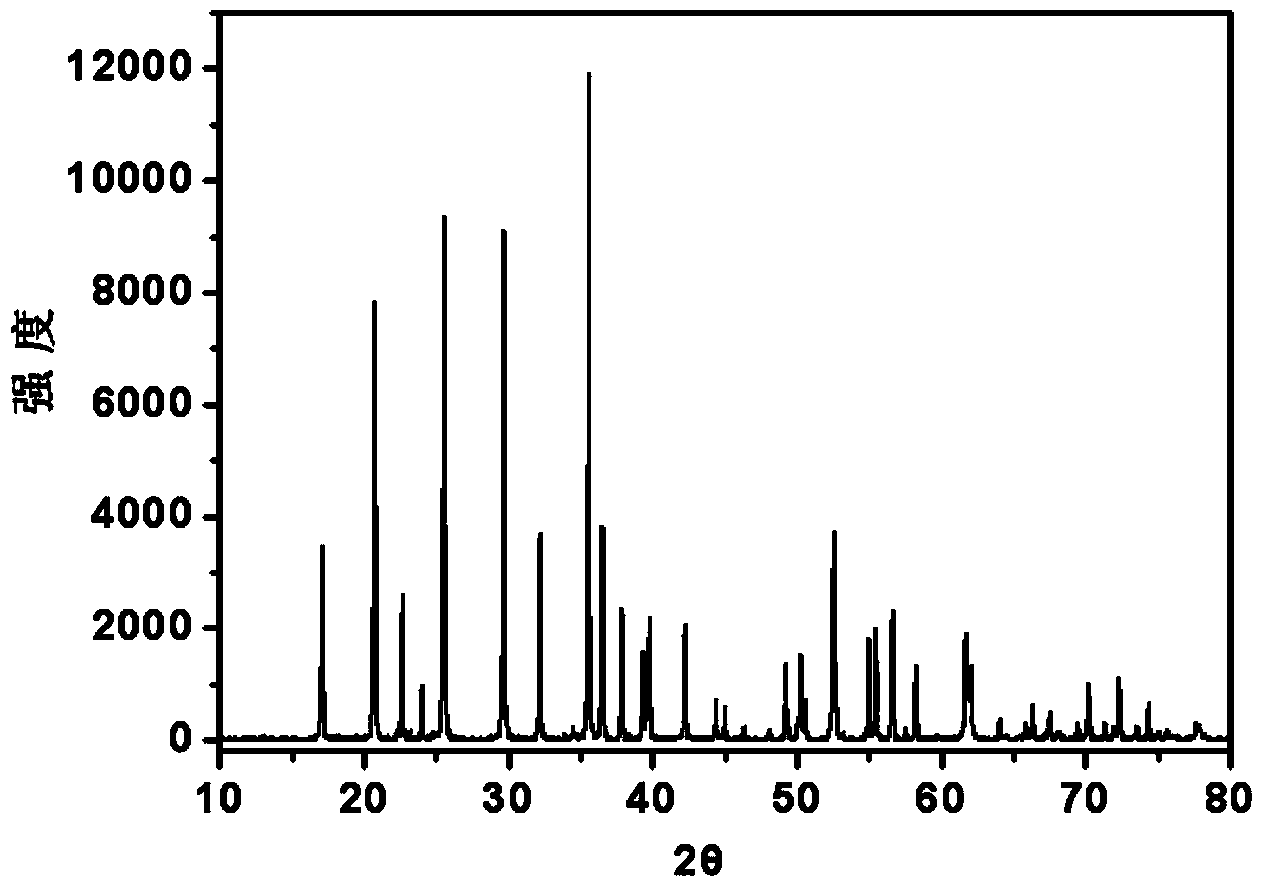
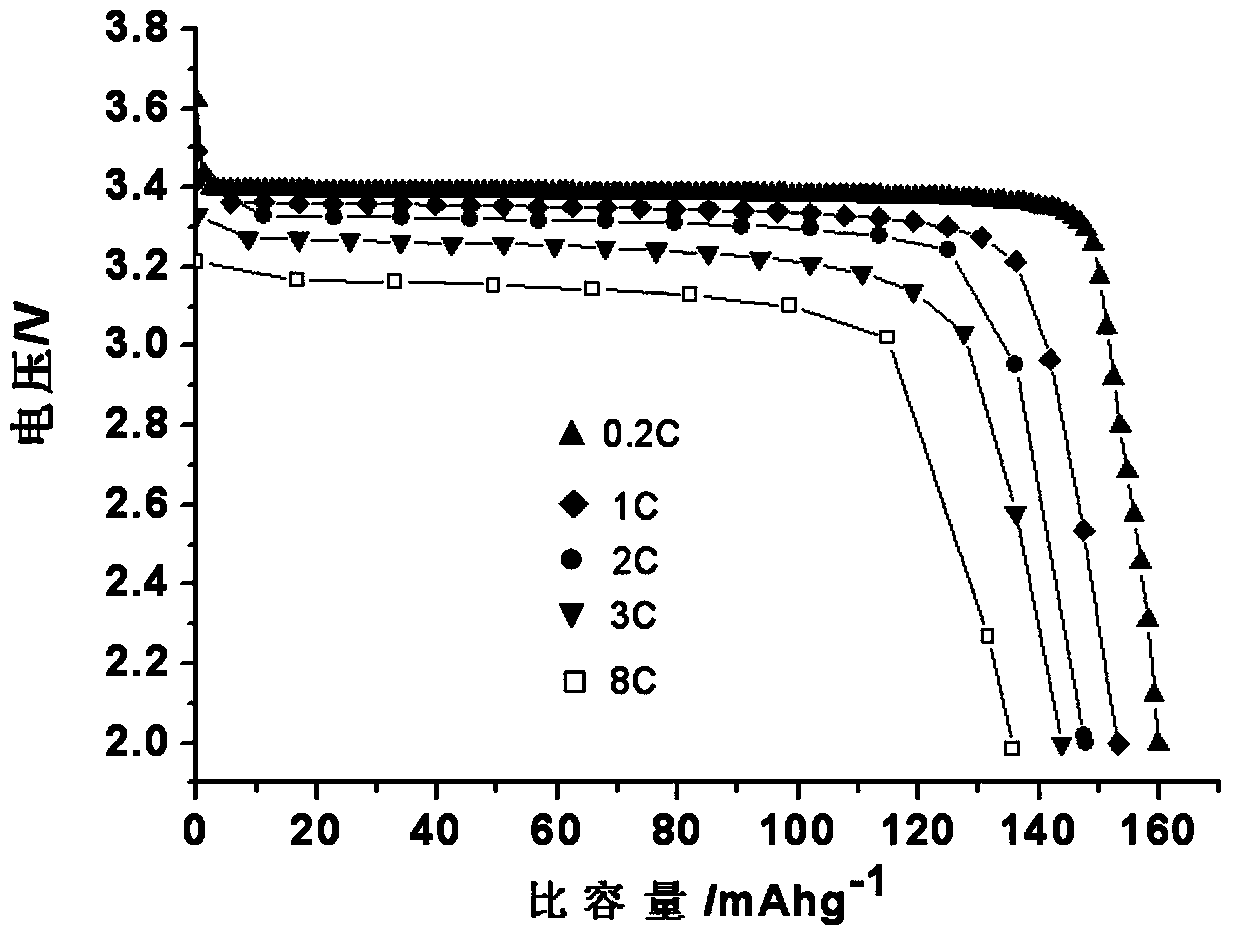
Method for preparing lithium phosphate/carbon-coated lithium iron phosphate composite material
A technology of carbon-coated lithium iron phosphate and composite materials, which is applied to electrical components, electrochemical generators, battery electrodes, etc. Problems such as poor consistency, to achieve the effect of improving electronic conductivity, high conductivity, and high performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]Weigh 111.16g of lithium carbonate, 539.41g of ferrous oxalate, and 345.36g of ammonium dihydrogen phosphate according to the molar ratio of lithium, iron, and phosphorus to 1:1:1 for the first mixing. Add ammonium dihydrogen phosphate, lithium carbonate, and ferrous oxalate to a 10L blue grinder with 3kg of ethanol in turn every half hour, at a speed of 1500r / min, and grind for 2 hours until the particle size reaches D50<4um. The material is introduced into the sand mill, and after the sand grinding particle size reaches 300-400nm, the slurry is poured into a tray and placed in a fume hood for static drying, and then crushed after complete drying. Under a nitrogen atmosphere, the crushed material was placed in a tube furnace for the first sintering at a sintering temperature of 600°C and kept at a constant temperature for 2 hours. Add 74.14g of the pre-calcined product to a 2L blue grinder containing 1000ml of ethanol for the second mixing at a speed of 1000r / min. After...
experiment example 2
[0037] Lithium hydroxide, iron oxide, and diammonium hydrogen phosphate were weighed for the first mixing according to the molar ratio of lithium, iron, and phosphorus elements being 1:0.9:0.95. Add diammonium hydrogen phosphate, lithium hydroxide, and iron oxide to a 10L blue grinder filled with 3kg of methanol in sequence every half hour, at a speed of 1500r / min, and grind for 2 hours until the particle size reaches D50<4um. The material is introduced into the sand mill, and after the sand grinding particle size reaches 300-400nm, the slurry is poured into a tray and placed in a fume hood for static drying, and then crushed after complete drying. Under a helium atmosphere, the crushed material was placed in a tube furnace for the first sintering at a sintering temperature of 500 degrees for 2 hours at a constant temperature. Add 71.78g of the pre-calcined product to a 2L blue grinder containing 1000ml of ethanol for the second mixing at a speed of 1000r / min. After grinding f...
experiment example 3
[0040] Weigh lithium acetate, iron citrate, and phosphoric acid for the first mixing according to the molar ratio of lithium, iron, and phosphorus elements being 1:0.97:0.98. Add phosphoric acid, lithium acetate, and ferric citrate to a 10L blue grinder filled with 3kg of acetone in turn every half hour at a speed of 1500r / min, and grind for 2 hours until the particle size reaches D50<4um, then import the slurry into the sand In the mill, after the sand grinding particle size reaches 300-400nm, pour the slurry into a tray and place it in a fume hood for drying, and then crush it after drying completely. Under an argon atmosphere, the crushed material was placed in a tube furnace for the first sintering at a sintering temperature of 400°C and kept at a constant temperature for 10 hours. Add 69.41g of the pre-calcined product to a 2L blue grinder containing 1000ml of N-methyl-2-pyrrolidone for the second mixing at a speed of 1000r / min. After grinding for half an hour, calculate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com