Simple preparation method of vitamin B6
A vitamin and simple technology, applied in the field of medicine and biochemical industry, can solve the problems of environmental pollution, long production cycle, heavy product coloring, etc., and achieve the effect of shortening the process cycle, short reaction cycle, simple operation and environmental protection.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
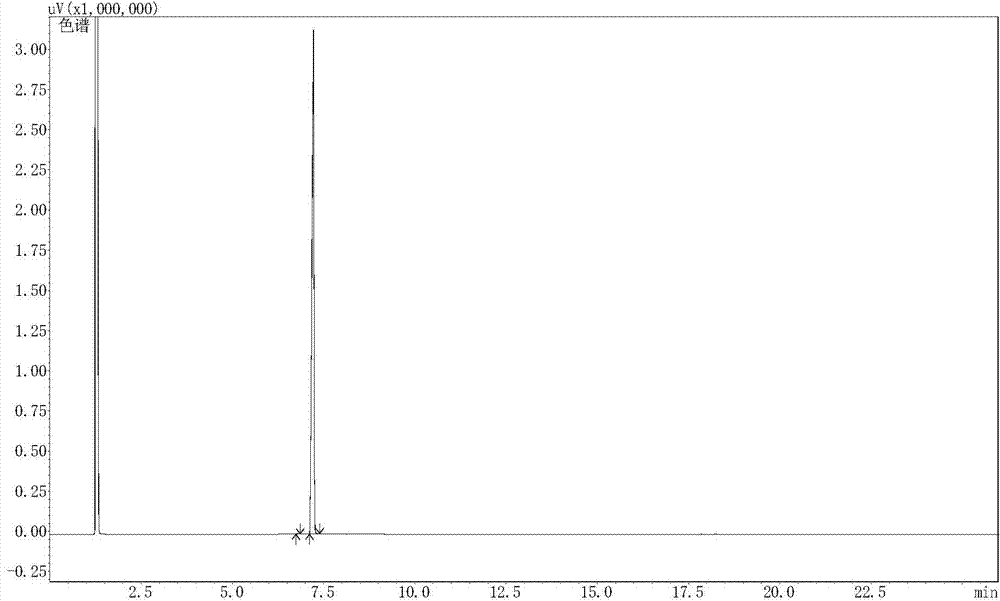
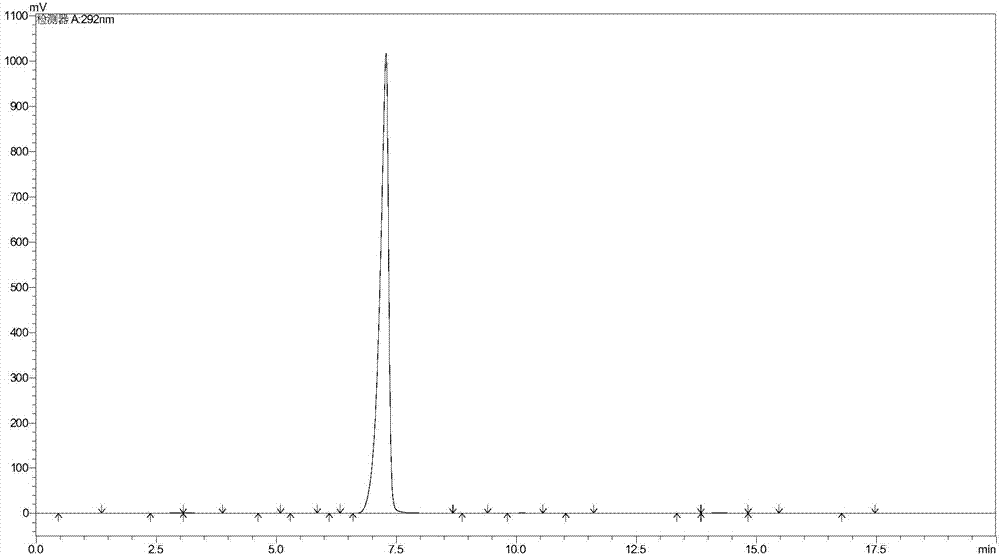
Image
Examples
Embodiment 1
[0041] Example 1: Preparation of 5-formyl-6-cyano-1,3-dioxepane
[0042] Add 200 grams of methanol, 83 grams (1.0 mole) of 3-cyanopropanal, 350 grams (3.5 moles) of 30% formaldehyde solution, 12 grams of zinc chloride, 15 grams of 30% hydrochloric acid, and nitrogen replacement in a 1000 milliliter reactor The second time, the temperature was raised to 80°C for 7 hours (the pressure was 2 atmospheres). Methanol was recovered, cooled, extracted three times with ethyl acetate (a total of 460 g was used), and the solvent was recovered to obtain 146.2 g of 5-formyl-6-cyano-1,3-dioxepane with a purity of 98.7% (GC). Yield 94.3%.
Embodiment 2
[0043] Example 2: Preparation of 5-formyl-6-cyano-1,3-dioxepane
[0044] Add 200 grams of 95% ethanol, 83 grams (1.0 moles) of 3-cyanopropionaldehyde, 350 grams (3.5 moles) of 30% formaldehyde solution, 15 grams of zinc chloride dihydrate, and 15 grams of 30% hydrochloric acid in a 1000 milliliter reactor , replaced with nitrogen three times, and heated to 80°C for 7 hours (pressure about 1.8 atmospheres). Methanol was recovered, cooled, extracted three times with ethyl acetate (500 g in total), and the solvent was recovered to obtain 145.4 g of 5-formyl-6-cyano-1,3-dioxepane with a purity of 98.3% (GC). The yield is 93.8%.
Embodiment 3
[0045] Example 3: Preparation of 5-formyl-6-cyano-1,3-dioxepane
[0046] Add 200 grams of methanol, 83 grams (1.0 moles) of 3-cyanopropanal, 350 grams (3.5 moles) of 30% formaldehyde solution, 12 grams of zinc acetate dihydrate, 15 grams of 30% hydrochloric acid, and replace with nitrogen in a 1000 ml reactor 3 times, the temperature was raised to 80°C for 7 hours (the pressure was 2 atmospheres). Methanol was recovered, cooled, extracted three times with ethyl acetate (a total of 450 g was used), and the solvent was recovered to obtain 143.4 g of 5-formyl-6-cyano-1,3-dioxepane with a purity of 98.9% (GC). Yield 92.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com