Colorimetric detection method of hydrogen peroxide
A hydrogen peroxide and detection method technology, which is applied in the direction of material analysis by observing the influence on chemical indicators, color/spectral characteristic measurement, and analysis by making materials undergo chemical reactions, can solve the problem of complex detection process and detection cost. High, high operating requirements, etc., to achieve the effect of low detection cost, good reproducibility, and mild enzyme-catalyzed reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
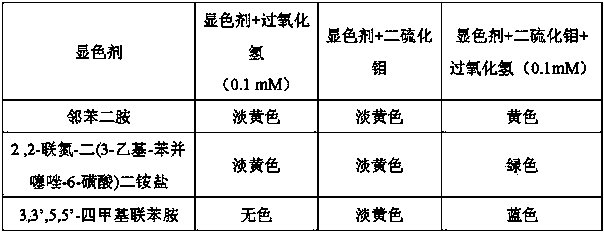
[0024] Detection of hydrogen peroxide in aqueous solution: Mix 0.2 mL hydrogen peroxide sample solution with 0.05 mL molybdenum disulfide solution (18 mg / L), 0.05 mL o-phenylenediamine (0.3 M) and 0.2 mL buffer solution (10 mM , pH 1) mixed, 12 minutes after the reaction can produce obvious color changes, semi-quantitative analysis can be carried out by visual colorimetry according to the color changes, or quantitative analysis can be carried out by measuring the absorbance at 450 nm wavelength by UV-spectrophotometry.
[0025] Standard colorimetric series: Mix 0.05 mL of molybdenum disulfide solution (18 mg / L), 0.05 mL of o-phenylenediamine (0.3 M) and 0.2 mL of buffer solution (10 mM, pH 1), and then add 0.2 mL of hydrogen peroxide standard solution of different concentrations, the concentrations are 0 μM, 5 μM, 20 μM, 40 μM, 60 μM, 80 μM, 100 μM, 150 μM, 200 μM, mix well, and react at 60°C for 30 min directly as a colorimetric standard series.
Embodiment 2
[0027] Detection of hydrogen peroxide in aqueous solution: mix 0.2 mL hydrogen peroxide sample solution with 0.05 mL molybdenum disulfide solution (18 mg / L), 0.05 mL 2,2-azino-bis(3-ethyl-benzo Thiazole-6-sulfonic acid) diammonium salt (20 mM) and 0.2 mL buffer solution (10 mM, pH 1) are mixed, and an obvious color change can be produced 12 minutes after the reaction, which can be determined by visual colorimetry according to the color change Semi-quantitative analysis, or quantitative analysis by measuring the absorbance at a wavelength of 405 nm by UV-spectrophotometry.
[0028] Standard colorimetric series: Mix 0.05 mL of molybdenum disulfide solution (18 mg / L), 0.05 mL of 2,2-azino-bis(3-ethyl-benzothiazole-6-sulfonic acid) diammonium salt (20 mM) and 0.2 mL buffer solution (10 mM, pH 1), after mixing, add 0.2 mL of hydrogen peroxide standard solution of different concentrations, the concentrations are 0 μM, 5 μM, 20 μM, 40 μM, 60 μM , 80 μM, 100 μM, 150 μM, and 200 μM we...
Embodiment 3
[0030] Detection of hydrogen peroxide in aqueous solution: mix 0.2 mL hydrogen peroxide sample solution with 0.05 mL molybdenum disulfide solution (18 mg / L), 0.05 mL 3,3',5,5'-tetramethylbenzidine ( 12 mM) mixed with 0.2 mL buffer solution (10 mM, pH 6.9), a significant color change can be produced 12 minutes after the reaction, which can be semi-quantitatively analyzed by visual colorimetry according to the color change, or by UV-spectrophotometry Quantitative analysis was carried out by measuring the absorbance at a wavelength of 652 nm; or adding 10 μL of 20% (V / V) sulfuric acid solution to terminate the reaction, and performing semi-quantitative analysis by visual colorimetry or using UV-spectrophotometry at a wavelength of 450 nm Absorbance was measured for quantitative analysis.
[0031]Standard colorimetric series: 0.05 mL of molybdenum disulfide solution (18 mg / L), 0.05 mL of 3,3',5,5'-tetramethylbenzidine (12 mM) and 0.2 mL of buffer solution (10 mM, pH 6.9), after m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com