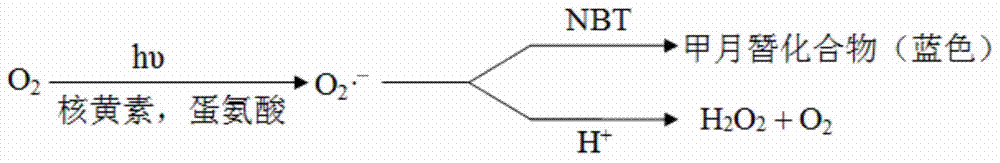
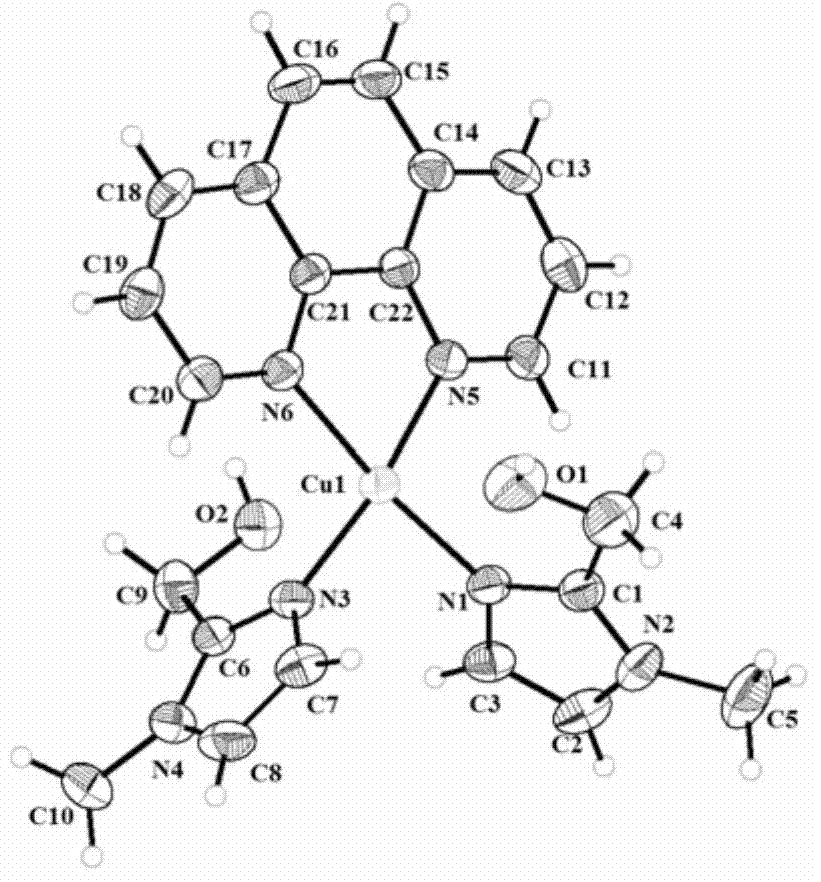
Imidazolyl copper complex and preparation method and application thereof
A technology of imidazolyl copper and ribimidazolyl copper is applied in the field of imidazolyl copper complexes and preparation thereof, and can solve the problems of difficulty in extracting superoxide dismutase and high extraction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] According to the present invention, the preparation method of the binuclear imidazole-based copper complex comprises: mixing N-methyl-2-hydroxymethylimidazole and copper perchlorate hexahydrate in a mixed solution of anhydrous methanol and absolute ethanol, And adjust the pH to 6.0-9.0, preferably under the condition that the pH value is 7-8, mix N-methyl-2-hydroxymethylimidazole and copper perchlorate hexahydrate for coordination reaction, filter the reaction product and crystallization.
[0027] According to the preparation method of the above-mentioned binuclear imidazole-based copper complex of the present invention, the pH of the coordination reaction has a crucial influence on the coordination reaction. When the pH is less than 6.0, the nitrogen atom on the HL will be protonated, so that it is difficult to combine with copper Ion coordination reaction, just can not obtain the imidazolium copper complex that the present invention provides; When pH is greater than 9...
Embodiment 1
[0045] Take 0.370g (1.0mmol) Cu(ClO 4 ) 2 ·6H 2 O, 0.224g (2.0mmol) N-methyl-2-hydroxymethylimidazole, 20mL mixed solvent of absolute methanol and absolute ethanol (the volume ratio of absolute methanol and absolute ethanol is 1:3), 20°C After stirring and dissolving under magnetic force, add triethylamine to adjust the pH of the solution to 8.0, and react at 80°C for 8 hours to obtain a dark blue clear solution, cool to room temperature, filter to remove insoluble matter, seal with plastic wrap, and evaporate to crystallize naturally, about 2 days later Dark blue cubic crystals A1 were precipitated with a yield of 62%.
[0046] A1 was detected, and the result of elemental analysis was C 20 h 30 Cl 2 N 8 o 12 Cu 2 : C, 30.92%; N, 14.35%; H, 4.12%, the theoretically calculated values are: C, 31.10%; N, 14.51%; H, 3.91%, indicating that the results of element analysis are consistent with the results of theoretical calculation. IR (KBrdisc, cm -1 ): 3439, 3138, 2375, ...
Embodiment 2
[0048] This example adopts the method of Example 1 to prepare the imidazole-based copper complex. The difference from Example 1 is that the N-methyl-2-hydroxymethylimidazole in this example is 1.8 mmol. In this example, dark blue cubic crystal A2 was obtained with a yield of 61%.
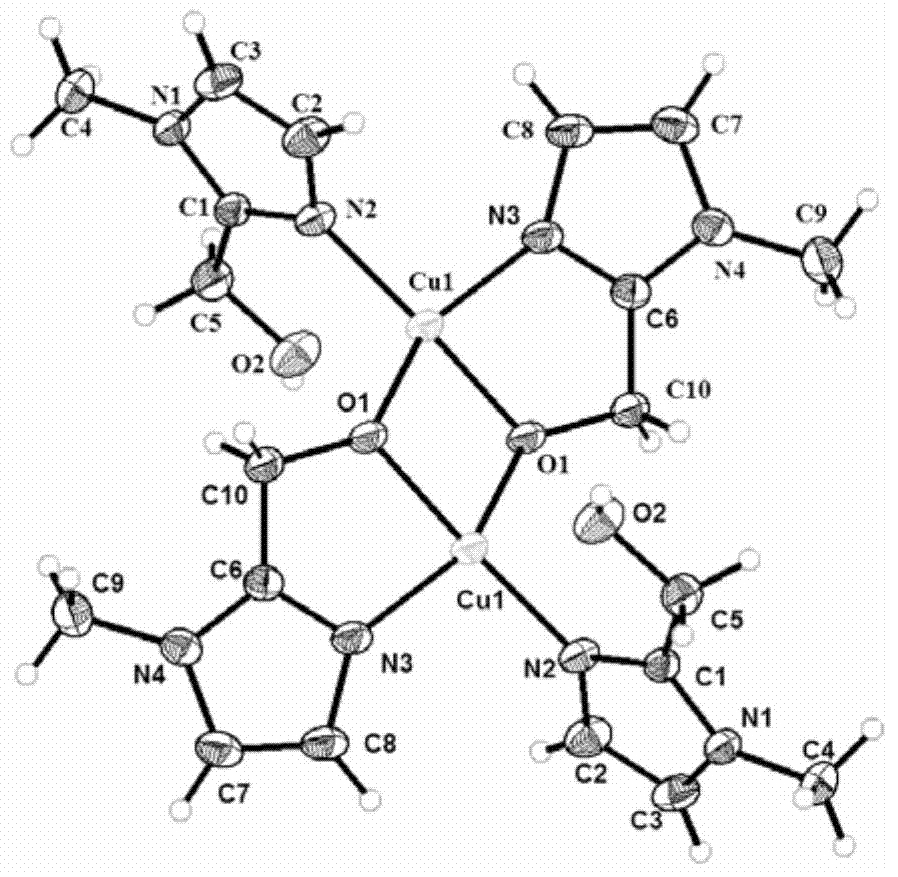
[0049] A2 was detected, and the result of elemental analysis was C 20 h 30 Cl 2 N 8 o 12 Cu 2 : C, 30.92%; N, 14.35%; H, 4.12%, the theoretically calculated values are: C, 31.10%; N, 14.51%; H, 3.91%, indicating that the results of element analysis are consistent with the results of theoretical calculation. IR (KBrdisc, cm -1 ): 3439, 3138, 2375, 2343, 2301, 1634, 1597, 1508, 1342, 1122, 1073, 752, 667, 626; single crystal diffractometer test obtained as figure 2 From the structure shown, it can be seen from infrared detection and single crystal diffraction detection that the structure of A2 is similar to the unique imidazole-bridged copper-zinc heterobinuclear structure of the SOD active ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com