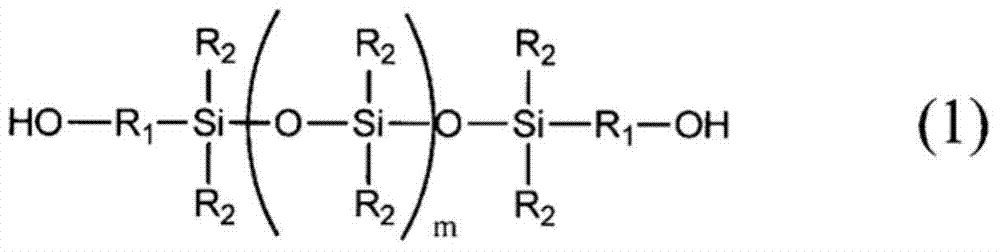
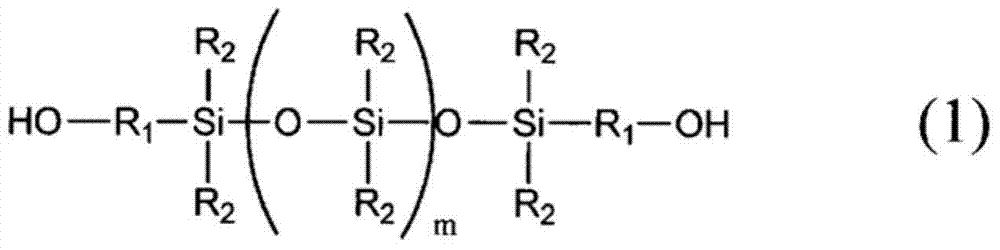
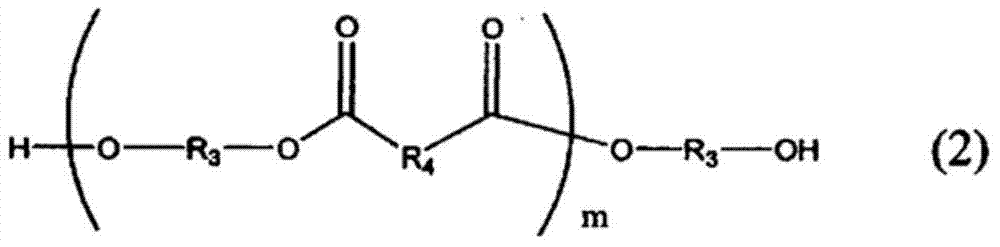
Curable resin composition for sealing optical semiconductor element and cured product thereof
A curable resin and optical semiconductor technology, applied in the direction of semiconductor devices, semiconductor/solid-state device parts, electrical components, etc., can solve problems such as poor operability, inability to achieve vulcanization resistance, poor thermal cycle resistance, etc., and achieve excellent pot life Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0298] Hereinafter, the present invention will be described in more detail through synthesis examples and examples. In addition, this invention is not limited to these synthesis examples and Examples. In addition, each physical property value in the synthesis example was measured by the following method.
[0299] Weight average molecular weight: It measured by the GPC method under the following conditions, and calculated the weight average molecular weight in terms of polystyrene.
[0300] Various conditions of GPC
[0301] Manufacturer: Shimadzu Corporation
[0302] Chromatographic column: guard column SHODEX GPC LF-G LF-804 (3 pieces)
[0303] Flow rate: 1.0ml / min
[0304] Column temperature: 40°C
[0305] Solvent used: THF (tetrahydrofuran)
[0306] Detector: RI (Differential Refractive Index Detector)
[0307] Epoxy equivalent: measured by the method described in JIS K-7236.
[0308] Acid value: Measured by the method described in JIS K-2501.
[0309] Viscosity: M...
Synthetic example 1
[0310] Synthesis Example 1 (Synthesis example of polysiloxane-skeleton epoxy resin (B) producing silanol-terminated silicone oil (e) and epoxy group-containing silicon compound (f) through a two-stage production process)
[0311] (Manufacturing process 1)
[0312] 394 parts of 2-(3,4-epoxycyclohexyl)ethyltrimethoxysilane and polydimethyldiphenylsiloxane having a silanol group with a molecular weight of 1700 (measured by GPC) were put into the reaction vessel (with 0.18 mole of phenyl group per mole of methyl group), 475 parts, 4 parts of 0.5% KOH methanol solution, 36 parts of isopropanol, and the temperature was raised to 75°C. After heating up, the reaction was carried out under reflux for 10 hours.
[0313] (Manufacturing process 2)
[0314] After adding 656 parts of methanol, 172.8 parts of a 50% distilled water methanol solution was added dropwise over 60 minutes, and the reaction was continued for 10 hours under reflux.
[0315]After the reaction was completed, methan...
Synthetic example 2
[0316] Synthesis Example 2 (Synthesis example of polysiloxane-skeleton epoxy resin (B) that produces silanol-terminated silicone oil (e) and epoxy group-containing silicon compound (f) through a two-stage production process)
[0317] (Manufacturing process 1)
[0318] 444 parts of 2-(3,4-epoxycyclohexyl)ethyltrimethoxysilane and polydimethyldiphenylsiloxane having a silanol group with a molecular weight of 1700 (measured by GPC) were put into the reaction vessel (0.18 moles of phenyl groups per mole of methyl group) 400 parts, 3.6 parts of 0.5% KOH methanol solution, 32.4 parts of isopropanol, heated up to 75°C. After heating up, the reaction was carried out under reflux for 10 hours.
[0319] (Manufacturing process 2)
[0320] After adding 480 parts of methanol, 194.4 parts of a 50% distilled water methanol solution was added dropwise over 60 minutes, and the reaction was continued for 10 hours under reflux.
[0321] After the reaction was completed, methanol was recovered...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com