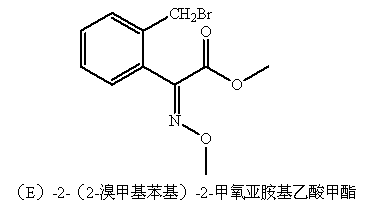
Preparation method of trifloxystrobin intermediate (E)-2-(2-bromomethyl phenyl)-2-methoxylimidomethyl acetate
A technology of methyl methoxyiminoacetate and bromomethyl phenyl, which is applied in the field of chemical synthesis, can solve the problems of atom economy and poor reaction selectivity, not meeting the needs of industrial use, and difficult treatment of bromine-containing wastewater. Achieve the effects of reducing corrosion and environmental pollution, reducing environmental pollution, and optimizing synthesis process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A preparation method of trifloxystrobin intermediate (E)-2-(2-bromomethylphenyl)-2-methoxyiminoacetic acid methyl ester, using o-toluic acid through chlorination, cyanation, The (E)-2-(2-methylphenyl)-2-methoxyiminoacetic acid methyl ester prepared by hydrolysis, esterification and oximation reaction is used as the raw material, and the specific steps are as follows:
[0022] (1) Bromination reaction: In a reactor equipped with 20.72g (E)-2-(2-methylphenyl)-2-methoxyiminoacetic acid methyl ester and 110.52g carbon tetrachloride, stir Add 1.04g of azobisisobutyronitrile, heat up to 62°C, then add a mixture of 22.4g of liquid bromine and 55.26g of carbon tetrachloride, and carry out a stirring reaction at a temperature of 62°C. When the color turns light yellow, add 8.29g of hydrogen peroxide with a mass fraction of 20%, raise the temperature to 65°C, and carry out the heat preservation and stirring reaction at a temperature of 65°C. In the reaction solution, (E)-2-(2- W...
Embodiment 2
[0025] A preparation method of trifloxystrobin intermediate (E)-2-(2-bromomethylphenyl)-2-methoxyiminoacetic acid methyl ester, using o-toluic acid through chlorination, cyanation, The (E)-2-(2-methylphenyl)-2-methoxyiminoacetic acid methyl ester prepared by hydrolysis, esterification and oximation reaction is used as the raw material, and the specific steps are as follows:
[0026] (1) Bromination reaction: In a reactor containing 41.11g (E)-2-(2-methylphenyl)-2-methoxyiminoacetic acid methyl ester and 276.31g carbon tetrachloride, stir Add 1.24g of benzoyl peroxide at the same time, heat up to 60°C, then add a mixture of 35.2g of liquid bromine and 138.15g of carbon tetrachloride, and carry out heat preservation and stirring reaction at a temperature of 60°C. The color of the solution to be reacted is When it turns light yellow, add 12.43g of hydrogen peroxide with a mass fraction of 25%, raise the temperature to 68°C, and carry out heat preservation and stirring reaction at...
Embodiment 3
[0029]A preparation method of trifloxystrobin intermediate (E)-2-(2-bromomethylphenyl)-2-methoxyiminoacetic acid methyl ester, using o-toluic acid through chlorination, cyanation, The (E)-2-(2-methylphenyl)-2-methoxyiminoacetic acid methyl ester prepared by hydrolysis, esterification and oximation reaction is used as the raw material, and the specific steps are as follows:
[0030] (1) Bromination reaction: In a reactor equipped with 20.72Kg (E)-2-(2-methylphenyl)-2-methoxyiminoacetic acid methyl ester and 179.59Kg carbon tetrachloride, stir Add 1.24Kg of benzoyl peroxide, heat up to 65°C, then add a mixture of 25.6Kg of liquid bromine and 89.80Kg of carbon tetrachloride, and carry out insulation and stirring reaction at a temperature of 65°C. The color of the solution to be reacted When it turns light yellow, add 10.36Kg of hydrogen peroxide with a mass fraction of 30%, raise the temperature to 75°C, and carry out heat preservation and stirring reaction at a temperature of 75...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com