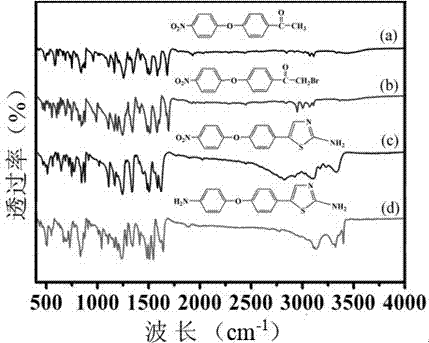
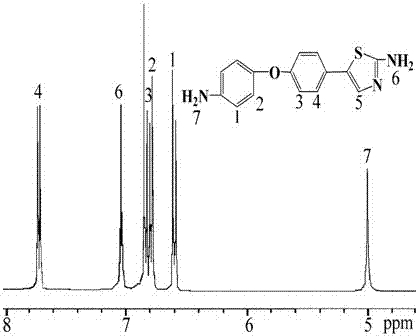
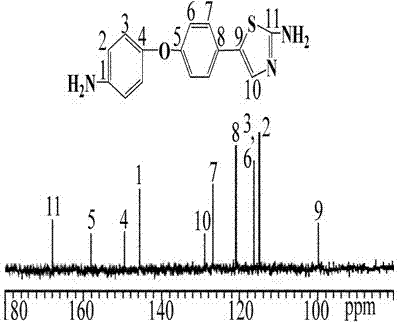
Preparation method of diamine compound containing thiazole ring
An amine compound, thiazole ring-containing technology, applied in the field of polymer synthesis, can solve the problems affecting the overall yield of the process route, long reaction time, complicated reaction device, etc., avoiding column chromatography and multiple recrystallization purification steps, Simplify the difficulty and technical requirements of process operation, simplify the effect of reaction process and device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] ①Put 45.00 g (C 8 h 8 o 2 , 136.15g / mol, 0.33 mol) p-Hydroxyacetophenone, 50.25 g (K 2 CO 3 , 138.21g / mol, 0.36 mol) anhydrous potassium carbonate, 54.67 g (C 6 h 4 ClNO 2 , 157.55g / mol, 0.35 mol) p-chloronitrobenzene and 150 mL DMAc, under continuous stirring, the reaction temperature was raised to 130-135°C to reflux the reaction mixture. After about 12 hours of reaction time, TLC followed the reaction process. After the reaction was completed, the reaction mixture was poured into 500 mL ice-water mixture (mass ratio 1:1), and a brownish-yellow precipitate precipitated out. Filter, wash the filter cake with water and ethanol successively, and put it into a vacuum drying oven at 40° C. to dry overnight. The crude product was recrystallized from ethanol to obtain 68.02 g of light yellow 4-(4'-nitrophenoxy)-acetophenone crystals. Yield: 80%. Melting point: 81~82℃;
[0022] ② Add 257.24 g (C 14 h 11 NO 4 , 257.24 g / mol, 1mol) 4-(4'-nitrophenoxy)-acetophenone ...
Embodiment 2
[0026] ①Put 40.85 g (C 8 h 8 o 2 , 136.15g / mol, 0.30 mol) p-Hydroxyacetophenone, 37.32 g (K 2 CO 3 , 138.21g / mol, 0.27mol) anhydrous potassium carbonate, 47.27 g (C 6 h 4 ClNO 2 , 157.55g / mol, 0.30 mol) p-chloronitrobenzene and 180 mL DMAc, under continuous stirring, the reaction temperature was raised to 135-140°C to reflux the reaction mixture. After about 10 hours of reaction time, TLC followed the reaction progress. After the reaction was completed, the reaction mixture was poured into 500 mL ice-water mixture (mass ratio 1:1), and a brownish-yellow precipitate precipitated out. Filter, wash the filter cake with water and ethanol successively, and put it into a vacuum drying oven at 40° C. to dry overnight. The crude product was recrystallized from ethanol to obtain 60.19 g of pale yellow 4-(4'-nitrophenoxy)-acetophenone crystals. Yield: 78%. Melting point: 81-82°C;
[0027] ②Add 514.48 g (C 14 h 11 NO 4 , 257.24 g / mol, 2mol) 4-(4'-nitrophenoxy)-acetophenone ...
Embodiment 3
[0031] ①Put 27.23 g (C 8 h 8 o 2 , 136.15g / mol, 0.20mol) p-Hydroxyacetophenone, 24.88 g (K 2 CO 3 , 138.21g / mol, 0.18 mol) anhydrous potassium carbonate, 31.51 g (C 6 h 4 ClNO 2 , 157.55g / mol, 0.20 mol) p-chloronitrobenzene and 70 mL DMAc under continuous stirring, the reaction temperature was raised to 140°C to reflux the reaction mixture. After about 12 hours of reaction time, TLC followed the reaction progress. After the reaction was completed, the reaction mixture was poured into 300 mL of ice-water mixture (mass ratio 1:1), and a brownish-yellow precipitate precipitated out. Filter, wash the filter cake with water and ethanol successively, and put it into a vacuum drying oven for 40 o C dried overnight. The crude product was recrystallized from ethanol to obtain 39.10 g of light yellow 4-(4'-nitrophenoxy)-acetophenone crystals. Yield: 76%. Melting point: 81-82 ℃;
[0032] ② Add 257.24 g (C 14 h 11 NO 4 , 257.24 g / mol, 1mol) 4-(4'-nitrophenoxy)-acetophenone a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com