Synthesis method of ticagrelor key intermediate
A technology of ticagrelor and a synthesis method, applied in the field of medicine, can solve problems such as no intermediate synthesis information is found, and achieve the effects of improving yield, simplifying process and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
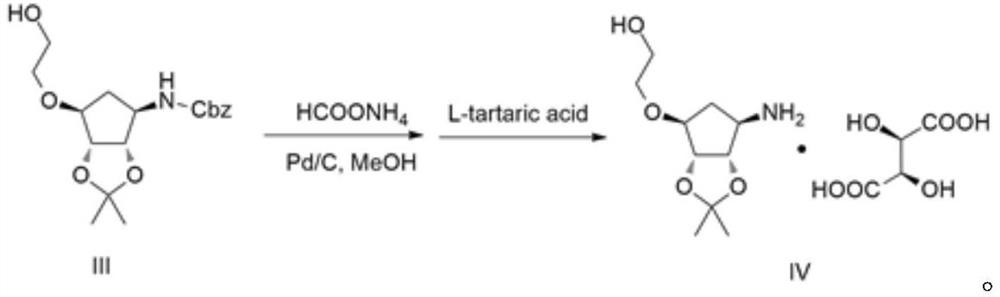
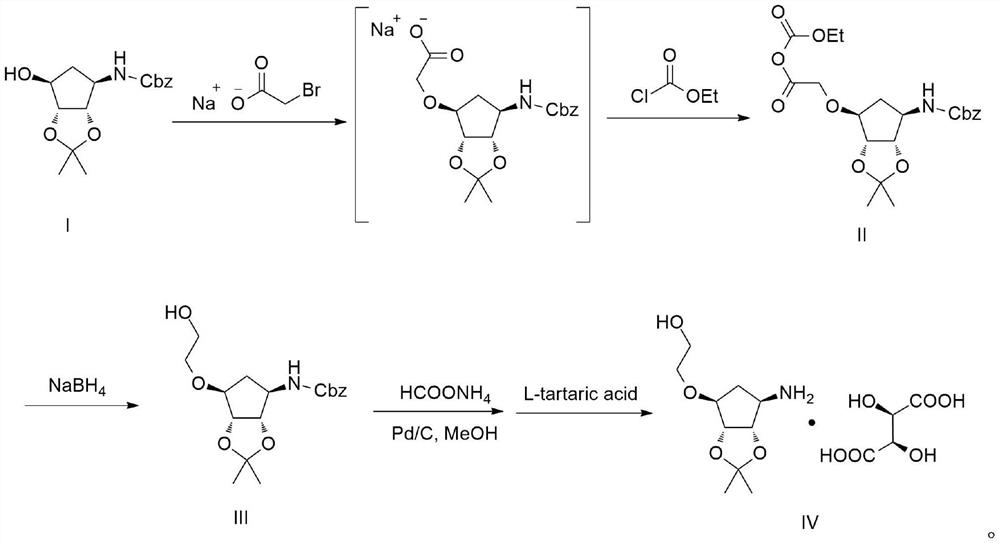
[0035]Example 1: Preparation of Compound III
[0036]100 g of compound I and 800 ml of tetrahydrofuran were inserted into 2L four-mouth bottle, and 40.1 g of tert-butoxide potassium t-butoxide was added to the bottle 1 to 0 to 10 ° C, and stirring was stirred after 15 minutes after addition. EtOAc EtOAc m....................................................................... .. The temperature was controlled at 10 to 20 ° C for 1 h, and then 29.7 g of sodium borohydride was added to 0 to 10 ° C. After the holding of 0 to 10 ° C for 4 h, the reaction of 30% acetic acid solution was quenched, pH to 4 to 5, and the tetrahydrofuran was concentrated under reduced pressure, then 500 ml of water was added and ethyl acetate (200 ml × 3), combined with organic phase It was also washed once with 200 ml of saturated saline, dried over anhydrous sodium sulfate to give 93.1 g of pale yellow oily compound III, and the yield was 81.39%.
[0037]MS: m / z = 352 (m + h+)
Embodiment 2
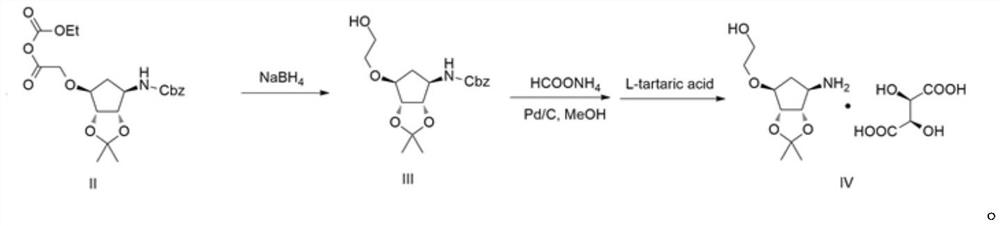
[0038]Example 2: Preparation of Compound III
[0039]100 g of compound I and 800 ml of tetrahydrofuran were inserted into 2L four-mouth bottles, and 68.8 g of t-butyl sodium t-butoxide was added to the bottle 1 to 0 to 10 ° C, and stirring was stirred after 15 minutes after addition. 4.9.5 g of bromoacetic acid was added to the bottle 1, and then stirred after 2 h after completion, then warmed to 10 to 20 ° C to the bottle 1 to slowly add 42.2 g of chloroformate. The temperature controlled 10 to 20 ° C was stirred for 1 h, then 30.9 g of sodium borohydride was added to 0 to 10 ° C. After the holding of 0 to 10 ° C for 4 h, the reaction of 30% acetic acid solution was quenched, pH to 4 to 5, and the tetrahydrofuran was concentrated under reduced pressure, then 500 ml of water was added and ethyl acetate (200 ml × 3), combined with organic phase It was washed once with 200 ml of saturated saline, dried over anhydrous sodium sulfate to give 81.7 g of yellow oily compound III, and the yiel...
Embodiment 3
[0041]Example 3: Preparation of Compound III
[0042]100 g of compound I and 800 ml of tetrahydrofuran were inserted into 2L four-mouth bottle 1, and 7.8 g of sodium hydrogen fluid was added to the bottle 1 to 0 ~ 5 ° C, and then stirred at 0.5 h after completion of the addition, then the temperature control 0 ~ 10 ° C 57.3 g of sodium bromoate was added in the bottle 1, and then stirred for 2 h after completion, then warmed to 10 ~ 20 ° C to the bottle 1 to slowly add 38.7 g of olycetate. The temperature was controlled at 10 to 20 ° C for 1 h, and then 24.8 g of sodium borohydride was added to 0 to 10 ° C. After the holding of 0 to 10 ° C for 4 h, the reaction of 30% acetic acid solution was quenched, pH to 4 to 5, and the tetrahydrofuran was concentrated under reduced pressure, then 500 ml of water was added and ethyl acetate (200 ml × 3), combined with organic phase It was also washed once with 200 ml of saturated saline, dried over anhydrous sodium sulfate to give 90.1 g of a pale ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com