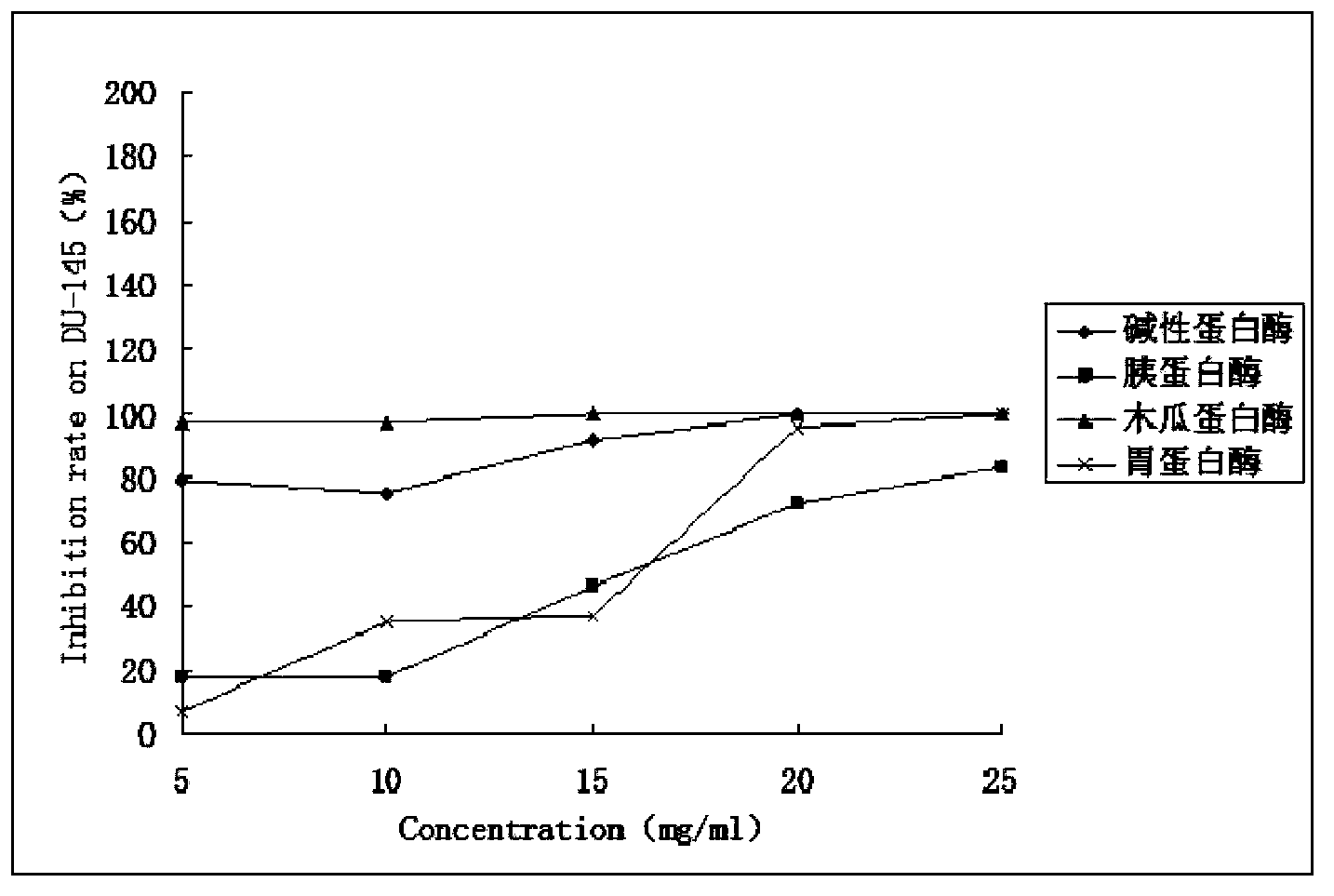
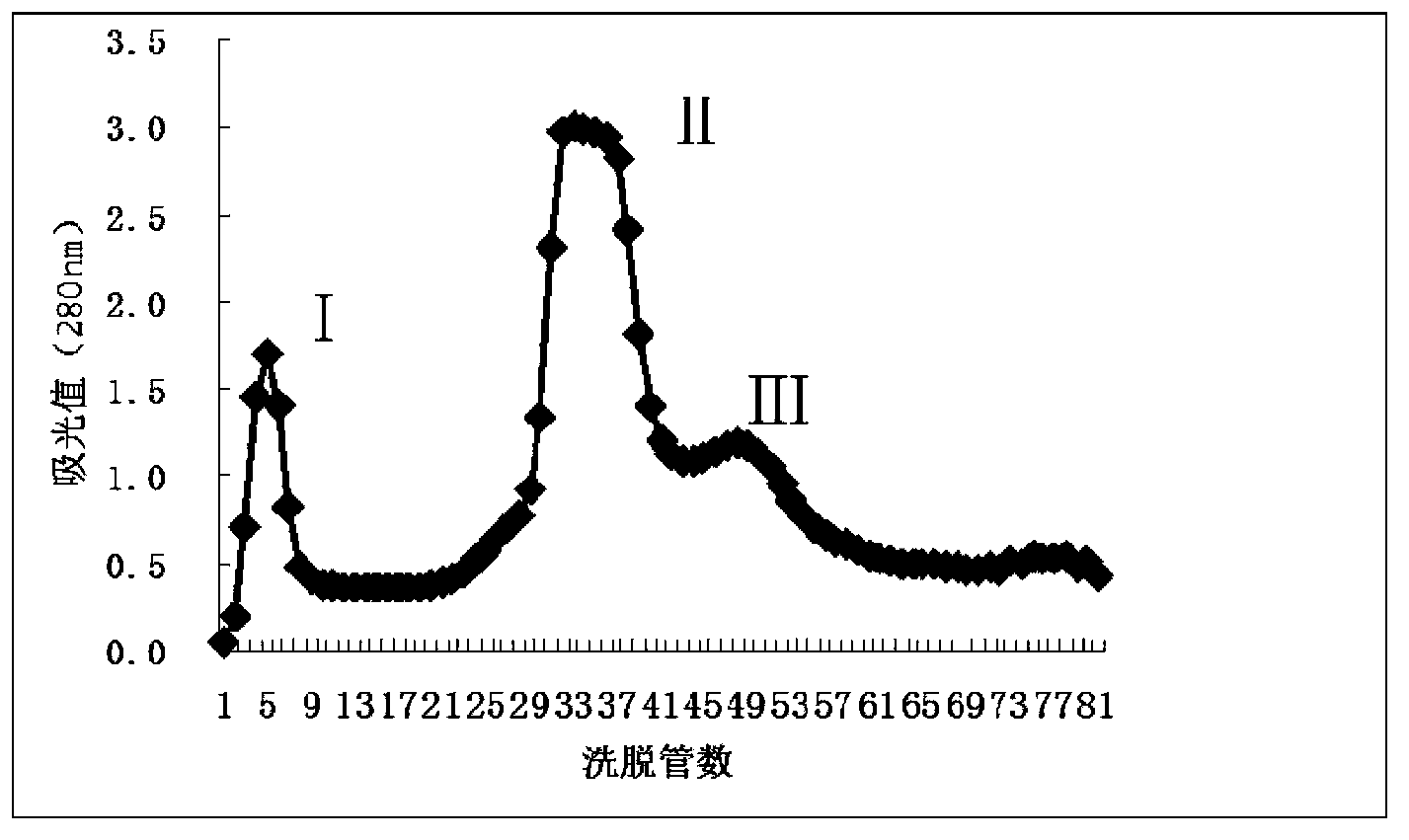
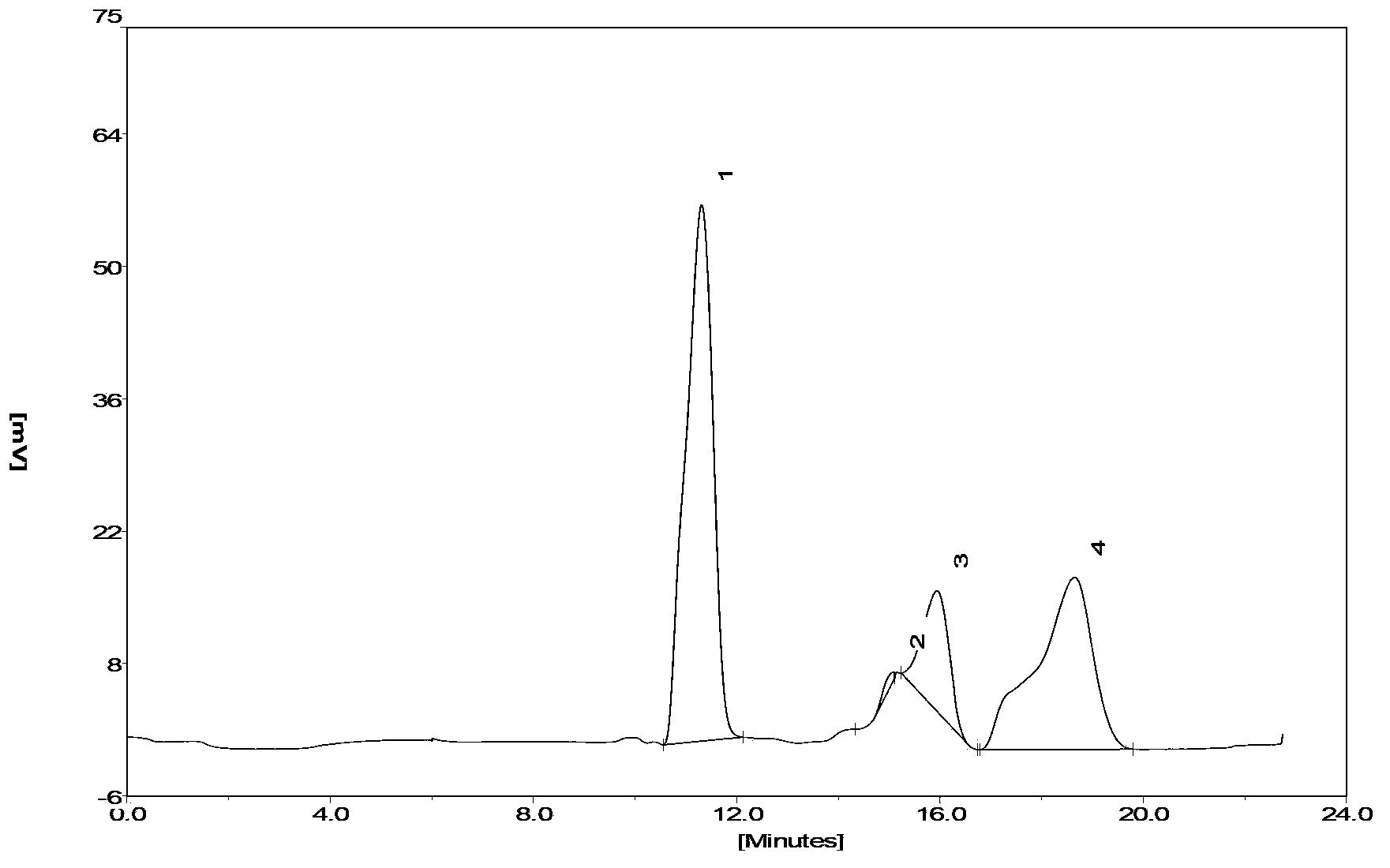
Preparation method and application of sinonovacula constricta enzymolysis polypeptide
A technology of enzymatic hydrolysis and razor clam, which is applied in the field of preparation of enzymatic hydrolyzed polypeptides of razor clam, achieving the effects of high sensitivity, good stability, and few side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] The present invention will be further described in detail below in conjunction with the accompanying drawings and embodiments.
[0032] 1. Materials and Instruments
[0033] 1.1 Main materials and reagents
[0034] Hanging razor clams were purchased from Nanzhen Market, Zhoushan City, Zhejiang Province;
[0035] Fetal bovine serum (FBS), Hangzhou Sijiqing Biological Products Co., Ltd.;
[0036] HamF12 medium, Gibco company;
[0037] Trypsin, American SIGMA company;
[0038] Penicillin, streptomycin, Shandong Lukang Pharmaceutical Co., Ltd.;
[0039] Dextran gel Sephadex G-25, Beijing Asia Pacific Hengxin Biotechnology Co., Ltd.;
[0040] Pepsin, papain, alkaline protease, Asia-Pacific Hengxin Biotechnology Co., Ltd.;
[0041] MTT was purchased from Sigma, USA;
[0042] Dimethyl sulfoxide (DMSO) was purchased from American AMRESCO;
[0043] The rest of the reagents were analytically pure.
[0044] 1.2 Main instruments
[0045] DS-1 high-speed tissue grinder, Shan...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com