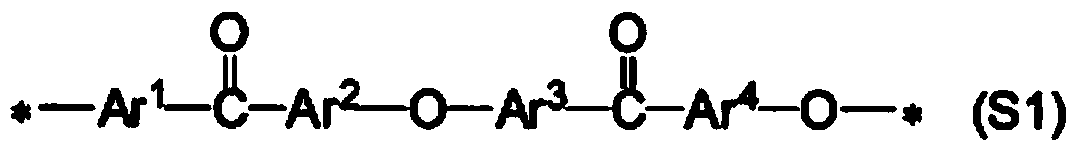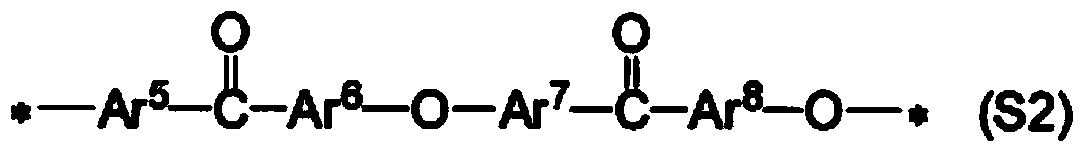Molded article of polymer electrolyte composition and solid polymer type fuel cell using same
A polymer electrolyte and molded body technology, applied in the direction of solid electrolyte fuel cells, fuel cells, fuel cell parts, etc., can solve the problems of long-term durability deterioration, proton conductivity increase, membrane rupture, etc., and achieve excellent physical Excellent effect of durability, high output, mechanical strength and chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0257] The present invention will be described more specifically through examples below, but the present invention is not limited thereto. In addition, the measurement conditions of each physical property are as follows. In addition, although the chemical structural formula is inserted in this Example, this chemical structural formula is inserted for the convenience of a reader's understanding, and is not limited to them.
[0258] (1) Ion exchange capacity
[0259] It measures by the neutralization titration method described in following (1)-(4). Measured 3 times, take their average value.
[0260] (1) After wiping off the moisture on the surface of the electrolyte membrane that had been proton-substituted and sufficiently washed with pure water, it was vacuum-dried at 100° C. for 12 hours or more, and the dry weight was determined.
[0261] (2) 50 mL of a 5% by weight sodium sulfate aqueous solution was added to the electrolyte, and the solution was left to stand for 12 ho...
Synthetic example 1
[0359] Synthesis of 2,2-bis(4-hydroxyphenyl)-1,3-dioxolane (K-DHBP) represented by the following general formula (G1)
[0360]
[0361] 49.5 g of 4,4'-dihydroxybenzophenone, 134 g of ethylene glycol, 96.9 g of trimethyl orthoformate, and 0.50 g of p-toluenesulfonic acid monohydrate were added to a 500 mL flask equipped with a stirrer, a thermometer, and a distillation tube , to dissolve them. Then keep stirring at 78-82° C. for 2 hours. Furthermore, the temperature was gradually raised to an internal temperature of 120° C., and the distillation of methyl formate, methanol, and trimethyl orthoformate was completely stopped. The reaction liquid was cooled to room temperature, and then the reaction liquid was diluted with ethyl acetate, and the organic layer was washed with 100 mL of 5% potassium carbonate aqueous solution, and after liquid separation, the solvent was distilled off. 80 mL of dichloromethane was added to the residue to precipitate crystals, which were filtere...
Synthetic example 2
[0363] Synthesis of 3,3'-sodium disulfonate-4,4'-difluorobenzophenone represented by the following general formula (G2)
[0364]
[0365] 109.1 g of 4,4'-difluorobenzophenone (Aldrich reagent) was added to fuming sulfuric acid (50% SO 3 ) in 150 mL (Wako Pure Chemical Chemicals), react at 100°C for 10 hours. Then, it was poured into a large amount of water little by little, neutralized with NaOH, and then 200 g of common salt was added to precipitate the composite. The obtained precipitate was filtered and recrystallized from ethanol aqueous solution to obtain 3,3'-sodium disulfonate-4,4'-difluorobenzophenone represented by the general formula (G2). The purity is 99.3%. structure 1 H-NMR confirmed. Impurities were quantitatively analyzed by capillary electrophoresis (organic) and ion chromatography (inorganic).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com