Flurbiprofen double-layer osmotic pump controlled-release tablet and preparation method thereof
A technology of osmotic pump controlled release and flurbiprofen, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, and drug delivery. problems such as large fluctuations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
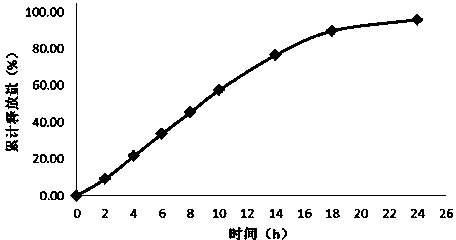
Embodiment 1
[0024] Tablet prescription:
[0025] Drug-containing layer:
[0026] Flurbiprofen 200mg
[0027] Polyoxyethylene 100mg
[0029] Mannitol 45mg
[0031] Boost layer:
[0032] Polyoxyethylene 200mg
[0034] Semi-permeable membrane envelope prescription:
[0035] Cellulose acetate 15mg
[0036] Polyethylene glycol 1.5mg
[0037] Solution prescription for dissolving coating material:
[0038] Acetone 500ml
[0039] Distilled water 15ml
[0040] Preparation Process:
[0041] Pass the prescribed amount of medicine, polyoxyethylene with a molecular weight of 200,000, sodium chloride, mannitol, and magnesium stearate through an 80-mesh sieve, and mix evenly; pass the prescribed amount of polyoxyethylene and sodium chloride with a molecular weight of 4,000,000 80-mesh sieve, mix evenly, use No. 12 punch twice to pressurize to get the tablet core. Dissolve cellulose acetate and poly...
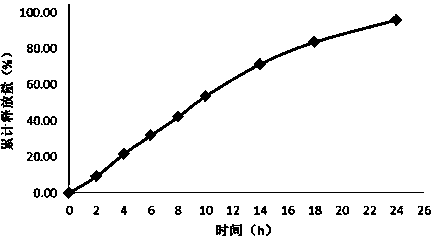
Embodiment 2
[0043] Tablet prescription:
[0044] Drug-containing layer:
[0045] Flurbiprofen 200mg
[0046]Polyvinylpyrrolidone 150mg
[0047] Mannitol 50mg
[0049] Boost layer:
[0050] Polyoxyethylene 150mg
[0051] Sodium chloride 50mg
[0052] Semi-permeable membrane envelope prescription:
[0053] Cellulose acetate 15mg
[0054] Polyethylene glycol 3mg
[0055] Solution prescription for dissolving coating material:
[0056] Acetone 500ml
[0057] Distilled water 15ml
[0058] Preparation Process:
[0059] Pass the prescribed amount of medicine, polyvinylpyrrolidone, mannitol, and magnesium stearate through a 80-mesh sieve, and mix evenly; pass the prescribed amount of polyoxyethylene and sodium chloride with a molecular weight of 7,000,000 through an 80-mesh sieve, mix evenly, and use Press the No. 12 punch twice to get the tablet core. Dissolve cellulose acetate and polyethylene glycol with an average molecular weight of 2000 in aceton...
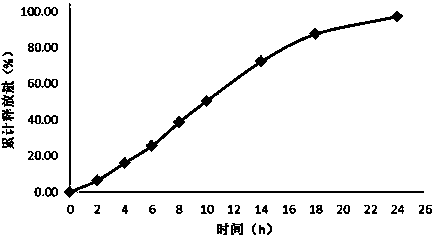
Embodiment 3
[0061] Tablet prescription:
[0062] Drug-containing layer:
[0063] Flurbiprofen 200mg
[0064] Sodium Alginate 200mg
[0065] Mannitol 50mg
[0066] Micronized silica gel 5mg
[0068] Boost layer:
[0069] Polyoxyethylene 150mg
[0070] Sodium chloride 50mg
[0071] Semi-permeable membrane envelope prescription:
[0072] Cellulose acetate 15mg
[0073] Polyethylene glycol 1mg
[0074] Solution prescription for dissolving coating material:
[0075] Acetone 500ml
[0076] Distilled water 10ml
[0077] Preparation Process:
[0078] Pass the prescribed amount of medicine, sodium alginate, mannitol, micropowder silica gel, and magnesium stearate through an 80-mesh sieve, and mix evenly; pass the prescribed amount of polyoxyethylene and sodium chloride with a molecular weight of 7,000,000 through an 80-mesh sieve, and mix Evenly, use the No. 12 punch to press twice to get the tablet core. Dissolve cellulose acetate and polyethylene gl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com